Predictive factors associated with gefitinib response in patients with advanced non-small-cell lung cancer (NSCLC)
Introduction
Gefitinib (Iressa) is an orally active small-molecule compound that inhibits the epidermal growth factor receptor (EGFR) tyrosine kinase (TK) by competing with adenosine triphosphate (ATP) at the ATP-binding site, which played a central role in advancing non-small-cell lung cancer (NSCLC) treatment over the last several years (1). Recent studies have shown that compared with counterparts, female cancer patients have favorable outcomes after gefitinib treatment (2-4) because females are more likely to have EGFR mutations (5-7) and most of females are non-smokers (8,9). However, in our clinical experience we did not observe that there was a gender difference in gefitinib response. This paper aims to identify the predictive factors that really contribute to gefitinib response in Chinese NSCLC patients including smoking status and gender.
Patients and methods
Patients’ characteristics
Thirty-three patients with advanced NSCLC patients, who were hospitalized in our hospital, were enrolled in this study. Among them, 21 (63.6%) were men and 12 (36.4) were women, with a median age of 59 years old (ranging from 29-76 years old). There were 20 (60.6%) non-smokers, 13 (39.4%) former/current smokers. Non-smokers are defined as those who reported smoked less than 100 cigarettes during their lifetime. Former smokers are defined as ever smokers who no longer smoked. Histological and/or cytological type was determined according to the World Health Organization/International Association for the Study of Lung Cancer classifications, with 23 (69.7%) adenocarcinomas, 9 (27.3%) squamous-cell carcinomas and 1 (3.0%) large-cell carcinomas. Current tumor stage was determined according to the TNM classification of malignant tumors. Two (6.1%) patients were classified as at Stage I, 3 (9.1%) at Stage II, 9 (27.3%) at Stage III and 19 (57.6%) at Stage IV. All cases with informed consent were received gefitinib monotherapy (250 mg/day orally) without regard to the gender, smoking history and EGFR mutation status. The drug response was evaluated according to the response evaluation criteria in solid tumors guidelines. Objective response is defined as patients with complete response or partial response, meanwhile progression-free survival (PFS) is defined as the time from the initial administration of chemotherapy to the earliest occurrence of disease progression or death from any cause. The protocol was approved by the Institutional Review Board, and fully informed written consent was obtained for all cases.
EGFR gene analysis
Before gefitinib monotherapy (250 mg/day), plasma was taken from each patient and EGFR gene typing was performed with mutant-enriched PCR assay (10). The majority of EGFR gene mutations consist of an in-frame deletion in exon 19 and a point mutation involving the replacement of leucine with arginine at codon 858 (L858R) in exon 21 (11). PCR products were detected with polyacrylamide gel electrophoresis analysis.
Statistical analysis
The differences in objective response (complete response + partial response) by each predictive factor (gender, smoking status and mutation status) were examined with the Fisher’s exact test or Pearson’s chi-square test. Multivariate analysis of the predictive factors, including gender (male vs. female), smoking history (smokers vs. non-smokers) and EGFR mutation (positive vs. negative) were conducted using the Cox regression model. All analysis was determined to be statistically significant where the P value was <0.05. Analyses were conducted using the SPSS 11.0.
Results
EGFR gene mutation analysis
Mutated EGFR gene including either EGFR gene exon 19 deletion or exon 21 mutation, or both. Plasma samples were collected from 33 patients and EGFR gene mutations occurred in 16 patients (48.5%). Seventeen patients (51.5%) were wild type gene type. No difference between the male and female in mutated gene incidence. But there was significant difference between non-smoker and smokers in mutated gene incidence (Table 1).
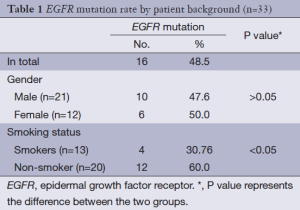
Full table
Gefitinib treatment response
Fifteen patients had objective response in 33 patients receiving gefitinib chemotherapy. Among them only 1 patient had complete response, 14 patients had partial response. Thirteen patients had stable disease, and 5 patients had progressed disease. The objective response rate (ORR) was 45.5% with PFS 3 (2.0-4.0) months. Among 16 patients with EGFR gene mutations, 1 case had complete response, 11 cases had partial response, while 2 cases had stable disease, 4 cases had no significant change. The ORR was 75.0%. Yet in the rest 17 patients without EGFR gene mutations, the ORR was 17.6% (Table 2). Univariate analysis showed that, for all 33 cases, compared with EGFR wild type gene group or smoker group, EGFR mutated gene group or non-smoker group seemed to be associated with improved gefitinib treatment response. Multivariate regression analysis showed that EGFR mutated gene other than non-smoker is the only independent predictive factor for ORR (Table 2).
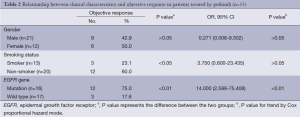
Full table
PFS in EGFR mutated gene group or non-smoker group was significantly longer than that in EGFR wild type gene group or smoker group (P<0.01). Cox-2 regression model analysis showed that both EGFR mutated gene and non-smoker are independent factors for PFS (Table 3).

Full table
Effect of smoking on gefitinib treatment response in NSCLC patients with EGFR mutated gene or wild-type gene
All 33 patients treated by gefitinib had been quadripartited into EGFR wild type gene/smoker group, EGFR wild type gene/non-smoker group, EGFR mutated gene/smoker group and EGFR mutated gene/non-smoker group. The ORR was increased from 11.1%, 25%, 50% to 83.3% correspondingly (Table 4). Twelve patients with EGFR mutated gene/non-smoker had longest PFS among the four groups (P<0.01) (Table 4).
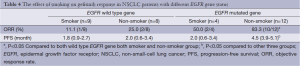
Full table
Discussion
We detected EGFR gene mutations in 33 NSCLC patients with mutant-enriched PCR assay and the positive mutation rate reached 45.5%, which was similar to the result of Scorpion ARMS technique established by Horiike et al. (12). Mutant-enriched PCR assay is a sensitive, specific, and inexpensive clinical well-developed technique (10). We further evaluated the correlation between EGFR gene mutation status and gefitinib response, as well as the predictive factors for gefitinib. In this study EGFR mutated gene patients treated by gefitinib had better ORR (75.0%) and PFS (4 months) than EGFR wild type gene patients (17.6% and 2 months). Multivariate regression analysis confirmed EGFR mutated gene was independent predictive factors for ORR and PFS.
Many retrospective studies have showed that EGFR mutated gene is more common in female than male patients (5-7). However, our study did not find the gender difference of EFGR mutation rate, as well as ORR and PFS. Is there any ethnic variation for gefitinib response? It is worth further studying in future.
Smoking status was an important predictive factor of gefitinib response in NSCLC patients. Our study suggested that, as regards ORR or PFS, NSCLC non-smoker patients have better sensitivity to gefitinib than smoker patients. Multivariate regression analysis confirmed that smoking was an independent predictive factor of PFS in NSCLC patients. Smoking significant shortened PFS in NSCLC EGFR mutated gene patients. However, smoking status did not significantly affect ORR and PFS in NSCLC patients with EGFR wild type gene.
Our results should be interpreted in the context of some limitations. First, our study used gefitinib which is no longer widely available in the United States after lack of survival benefit was reported in previously treated patients with non-selected NSCLC (13). However, some preplanned subgroup analyses revealed survival benefits in Asian and non-smoker patients (3). Gefitinib is still widely used in China. Second, more information on the gefitinib response is needed from the whole country with the highest lung cancer-related deaths rate in China. Third, our samples were just collected from a university hospital, which means that patients’ selection bias cannot be completely ruled out because urban patients may more easily exposed to industrial pollution compared with rural patients. Finally, our sample size was small (total 33 patients).
Conclusions
Despite limitations, our results indicated that EGFR mutation is an important predictive factor of gefitinib response in NSCLC patients, and smoking history will affect gefitinib response. This strategy has great potential to explain the gefitinib resistance but further basic research and clinical trials are urgently needed.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Sequist LV, Joshi VA, Jänne PA, et al. Response to treatment and survival of patients with non-small cell lung cancer undergoing somatic EGFR mutation testing. Oncologist 2007;12:90-8. [PubMed]
- Landi L, Cappuzzo F. Irreversible EGFR-TKIs: dreaming perfection. Transl Lung Cancer Res 2013;2:40-9.
- Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005;366:1527-37. [PubMed]
- Song Z, Yu X, He C, et al. Re-administration after the failure of gefitinib or erlotinib in patients with advanced non-small cell lung cancer. J Thorac Dis 2013;5:400-5. [PubMed]
- Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339-46. [PubMed]
- Kosaka T, Yatabe Y, Endoh H, et al. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res 2004;64:8919-23. [PubMed]
- Yang CH, Yu CJ, Shih JY, et al. Specific EGFR mutations predict treatment outcome of stage IIIB/IV patients with chemotherapy-naive non-small-cell lung cancer receiving first-line gefitinib monotherapy. J Clin Oncol 2008;26:2745-53. [PubMed]
- Zhao YY, Zhang Y, Zhao HY, et al. Predictive factors for response and survival of gefitinib-treated locally advanced or metastatic non-small cell lung cancer patients: a retrospective analysis of two phase II clinical trialsAi Zheng 2009;28:626-31.
- Satouchi M, Negoro S, Funada Y, et al. Predictive factors associated with prolonged survival in patients with advanced non-small-cell lung cancer (NSCLC) treated with gefitinib. Br J Cancer 2007;96:1191-6. [PubMed]
- He C, Liu M, Zhou C, et al. Detection of epidermal growth factor receptor mutations in plasma by mutant-enriched PCR assay for prediction of the response to gefitinib in patients with non-small-cell lung cancer. Int J Cancer 2009;125:2393-9. [PubMed]
- Xu CR, Lin JY, Wang Z, et al. Relationship of the curative effects of gefitinib on non-small cell lung carcinoma to gender and to epithelial growth factor receptor status. Zhonghua Yi Xue Za Zhi 2006;86:2606-10. [PubMed]
- Horiike A, Kimura H, Nishio K, et al. Detection of epidermal growth factor receptor mutation in transbronchial needle aspirates of non-small cell lung cancer. Chest 2007;131:1628-34. [PubMed]
- Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol 2008;26:2442-9. [PubMed]


