PARP inhibitor reduces proliferation and increases apoptosis in breast cancer cells
Introduction
Breast cancer is a major threat to women’s health and its incidence and fatality rate are gradually increasing (1-6). The pathogenesis of breast cancer is associated with genetic and environmental factors, as well as essential cellular pathways such as those controlling metabolisms, cell cycle progression, proliferation, and apoptosis (7,8).
Poly(ADP-ribose) polymerase (PARP) inhibitors target DNA repair defects in hereditary breast cancer. PARP inhibitors as monotherapy or in combination therapy have yielded promising results against different cancers in recent clinical trials (9). Olaparib is a novel PARP inhibitor that induces synthetic lethality in BRCA-deficient cells. PARP plays an important role in base excision repair of single-strand DNA breaks: inhibition of PARP results in the accumulation of single-strand DNA breaks, leading to the formation of double-strand breaks after stalling and collapse of the DNA replication fork (10). These double-strand breaks are usually repaired by the error-free homologous recombination repair pathway, of which the tumor-suppressor genes BRCA1 and BRCA2 are key components (11-15).
Paclitaxel, one of the most effective chemotherapeutic drugs, was isolated from the bark of the Pacific yew (Taxus brevifolia). The drug promotes and stabilizes microtubules and inhibits the late G2 and M phases of the cell cycle, thereby causing cell death. It is mainly used to treat lung, ovarian, and breast cancers (16,17).
Several studies have shown that paclitaxel or PARP inhibitors such as rucaparib, PJ34 and veliparib inhibit tumor cell proliferation and promote apoptosis (18-20). Only a few studies have addressed the efficacy of olaparib in breast cancer. Therefore, we designed a study to determine the efficacy of paclitaxel combined with olaparib and explore the mechanism of olaparib-induced apoptosis in breast cancer cells.
Materials and methods
Cell culture and reagents
Human breast cancer Bcap37 cells were purchased from the Cell Bank of the Shanghai Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences. The cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA). Paclitaxel and olaparib were purchased from Sigma (St. Louis, USA) and LC Laboratories (Woburn, MA, USA) and dissolved in 100% dimethyl sulfoxide (DMSO, Sigma, USA). Cells were treated with paclitaxel alone (50 µg/mL) or in combination with the PARP inhibitor olaparib at 100, 200, or 400 mg.
MTT assay
We used 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assays to measure survival in breast cancer Bcap37 cells treated with the PARP inhibitor. Bcap37 cells were digested and diluted to 2×105/mL; 100 µL of this cell suspension was seeded in 96-well culture plates (Falcon, Oxnard, CA, USA). After 24 h, the cells were divided into untreated, paclitaxel, and paclitaxel+100/200/400 mg olaparib groups. Cells were harvested by scraping and suspending in medium after 24-h treatment. MTT solution (100 µL, Promega, USA) was added and the plates were incubated at 37 °C and 5% CO2 for another 4 h. DMSO (200 µL) was added to dissolve the MTT crystals; the supernatant was removed by centrifugation and absorbance was measured at 560 nm. Treatments were performed in triplicate wells.
Annexin FITC-V/PI double staining and flow cytometry
Annexin V-fluorescein isothiocyanate/propidium iodide (Annexin FITC-V/PI) double staining and fluorescence microscopy were used to quantitate apoptosis. Adherent cells were trypsin-digested and collected. The cells were washed twice and resuspended in 500 µL binding buffer and 5 µL annexin FITC-V and then in 5 µL PI. Flow cytometry was performed to detect the DNA concentration and percentage of apoptotic cells.
Western blotting
Cultured Bcap37 cells were collected by trypsin digestion and washed twice with phosphate-buffered saline (PBS). Total cell protein was extracted, quantified by the Bio-Rad Dc protein quantitative assay, and separated by 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred to polyvinylidene fluoride (PVDF) membranes and incubated with primary antibody overnight at 4 °C with shaking. The membranes were washed several times with Tris-buffered saline/Tween-20 and incubated with horseradish peroxidase (HRP)-labeled secondary antibody for 1 h at room temperature with shaking. Band intensity was detected by ECL detection reagent (Amersham, UK) with β-actin as an internal control.
Statistical analysis
Data analysis was performed with SPSS 16.0 software (SPSS Inc., Chicago, IL, USA); results are presented as . Analysis of variance (ANOVA) was used for multiple comparisons and Student’s t-test was used to compare the mean when there was significant variation between the treatment and control groups. P<0.05 was considered statistically significant.
Results
PARP inhibitor suppressed Bcap37 cell growth
MTT cytotoxicity analysis was used to measure Bcap37 cell proliferation. Growth inhibition by paclitaxel alone or in combination with olaparib is shown in Figure 1. Survival decreased with increasing incubation time. Bcap37 cells treated with paclitaxel combined with various concentrations of olaparib (100, 200 or 400 mg) showed significantly greater inhibition than cells treated with paclitaxel alone (P<0.05); inhibition increased with increasing olaparib dose and treatment time (Table 1, P <0.01).
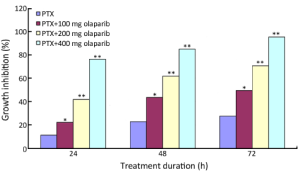
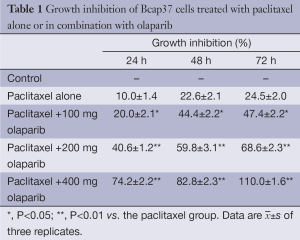
Full table
PARP inhibitors induced Bcap37 cell apoptosis
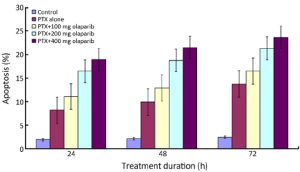
Apoptosis of Bcap37 was assessed by Annexin V/PI staining and fluorescence microscopy (Table 2, Figures 2,3). Apoptosis was induced by paclitaxel alone and in combination with olaparib. Combined treatment yielded significantly higher rates of apoptosis than paclitaxel alone (P<0.05) and the rates increased with time (P<0.01). Fluorescence micrographs showed that early and late apoptotic cells increased with treatment time. Olaparib inhibited Bcap37 cell proliferation and promoted apoptosis, and this effect enhanced in combination with paclitaxel.
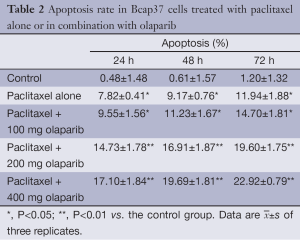
Full table

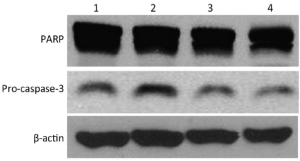
PARP inhibitors induced degradation of pro-caspase-3 and PARP in Bcap37 cells
Pro-caspase-3 and PARP were detected by Western blotting of Bcap37 cells treated with paclitaxel alone or in combination with olaparib (Figure 4). Pro-caspase-3 was degraded and PARP was cleaved into two fragments. Pro-caspase-3 and PARP degradation was induced by paclitaxel and enhanced by olaparib in a dose-dependent manner.
Discussion
Breast cancer is one of the most common malignancies in women; it is a complex and intrinsically heterogeneous disease, and its incidence and mortality continue to rise (21).
Olaparib is a potent oral PARP inhibitor with acceptable levels of toxicity and monotherapeutic activity in patients who have breast or ovarian cancer with a germline BRCA1 or BRCA2 mutation. The maximum tolerated dose and initial signs of efficacy in BRCA-deficient ovarian cancers have been reported. Tutt and colleagues evaluated the efficacy, safety and tolerability of olaparib alone in BRCA1- or BRCA2-mutated females with advanced breast cancer. The findings of their study provided robust evidence that supports the concept of PARP inhibition in BRCA-deficient breast cancers and showed a favorable therapeutic index for a novel targeted treatment strategy in patients with tumors genetically depleted of BRCA1-associated or BRCA2-associated DNA repair functions (22,23).
Many studies have addressed the anticancer efficacy of the plant-derived compound, paclitaxel. Paclitaxel is a broad-spectrum anticancer drug, providing therapeutic benefits for advanced cancers that are resistant to conventional therapeutic agents, and may exhibit significant curative effects for many clinical malignancies (24). There have been intensively used in clinical practices for paclitaxel since 1987 (25). Paclitaxel regulates microtubule stabilization, induces cell apoptosis and modifies immune functions. In particular, like taxinol which targets the microtubule system, paclitaxel promotes polymerization and inhibits degradation of the microtubules, thereby arresting cell cycle in G2/M stage and inducing tumor cell apoptosis. Paclitaxel is used to treat advanced ovarian cancer, breast cancer, non-small cell lung cancer, and esophageal cancer (14). Paclitaxel is one of the most effective drugs for the treatment of esophageal cancer, with a monotherapy efficiency of 32%. A number of phase I/II clinical trials have demonstrated the efficacy of paclitaxel in the treatment of locally advanced and metastatic esophageal cancer (26). In addition, gemcitabine and paclitaxel are reported in a study by Sandler as new cytotoxic agents which have both been used as single agents or in combination, especially with cisplatin, in the therapy of non-small cell lung cancer with encouraging outcomes (27). Paclitaxel improves therapeutic efficacy when used in combination with other anticancer drugs and has thus come into broad use. Chemotherapy aims to regulate cell growth and apoptosis to eliminate cancer cells. Paclitaxel, gefitinib, gemcitabine, and vinorelbine inhibit proliferation and induce apoptosis in breast cancer (28-34).
We assessed the proliferation and apoptosis of Bcap37 cells treated with paclitaxel alone or in combination with olaparib. The results suggested that PARP inhibitors and paclitaxel function by different mechanisms, but both inhibit tumor proliferation and promote apoptosis; in combination, they yield a synergistic effect. Pro-caspase-3 is a key mediator of apoptosis and PARP is the main substrate for caspase-3; both are important in DNA damage repair and apoptosis. Our study showed that increasing the dose of olaparib led to increased degradation of pro-caspase-3 and PARP, thus confirming that olaparib induces Bcap37 apoptosis. Combined paclitaxel and olaparib inhibited DNA damage repair and may provide an ideal drug option for chemotherapy, although additional research is needed to determine the mechanism of the actions.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Shaukat U, Ismail M, Mehmood N. Epidemiology, major risk factors and genetic predisposition for breast cancer in the Pakistani population. Asian Pac J Cancer Prev 2013;14:5625-9. [PubMed]
- Assi HA, Khoury KE, Dbouk H, et al. Epidemiology and prognosis of breast cancer in young women. J Thorac Dis 2013;5:S2-8. [PubMed]
- Li H, Beeghly-Fadiel A, Wen W, et al. Gene-environment interactions for breast cancer risk among Chinese women: a report from the Shanghai Breast Cancer Genetics Study. Am J Epidemiol 2013;177:161-70. [PubMed]
- Colditz GA. Epidemiology of breast cancer. Findings from the nurses’ health study. Cancer 1993;71:1480-9. [PubMed]
- Bearal V; Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 2003;362:419-27. [PubMed]
- Easton DF, Pooley KA, Dunning AM, et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature 2007;447:1087-93. [PubMed]
- Bertos NR, Park M. Breast cancer - one term, many entities? J Clin Invest 2011;121:3789-96. [PubMed]
- Russnes HG, Navin N, Hicks J, et al. Insight into the heterogeneity of breast cancer through next-generation sequencing. J Clin Invest 2011;121:3810-8. [PubMed]
- Lord CJ, Ashworth A. The DNA damage response and cancer therapy. Nature 2012;481:287-94. [PubMed]
- Amé JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays 2004;26:882-93. [PubMed]
- Gudmundsdottir K, Ashworth A. The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene 2006;25:5864-74. [PubMed]
- Tutt A, Bertwistle D, Valentine J, et al. Mutation in Brca2 stimulates error-prone homology-directed repair of DNA double-strand breaks occurring between repeated sequences. EMBO J 2001;20:4704-16. [PubMed]
- Moynahan ME, Chiu JW, Koller BH, et al. Brca1 controls homology-directed DNA repair. Mol Cell 1999;4:511-8. [PubMed]
- Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell 2001;7:263-72. [PubMed]
- Moynahan ME. The cancer connection: BRCA1 and BRCA2 tumor suppression in mice and humans. Oncogene 2002;21:8994-9007. [PubMed]
- Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer 2004;4:253-65. [PubMed]
- Ma P, Mumper RJ. Paclitaxel nano-delivery systems: a comprehensive review. J Nanomed Nanotechnol 2013;4:1000164. [PubMed]
- Ali M, Kamjoo M, Thomas HD, et al. The clinically active PARP inhibitor AG014699 ameliorates cardiotoxicity but does not enhance the efficacy of doxorubicin, despite improving tumor perfusion and radiation response in mice. Mol Cancer Ther 2011;10:2320-9. [PubMed]
- Magan N, Isaacs RJ, Stowell KM. Treatment with the PARP-inhibitor PJ34 causes enhanced doxorubicin-mediated cell death in HeLa cells. Anticancer Drugs 2012;23:627-37. [PubMed]
- Khan O A, Gore M, Lorigan P, et al. A phase I study of the safety and tolerability of olaparib (AZD2281, KU0059436) and dacarbazine in patients with advanced solid tumours. Br J Cancer 2011;104:750-5. [PubMed]
- Althuis MD, Dozier JM, Anderson WF, et al. Global trends in breast cancer incidence and mortality 1973-1997. Int J Epidemiol 2005;34:405-12. [PubMed]
- Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 2010;376:245-51. [PubMed]
- Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 2010;376:235-44. [PubMed]
- Tong Z, Zhong D. Antitumor mechanism of taxinol. Chin J Clin Rehabilitation 2006;27:125-7.
- Sun Y, Zhang X, Zhang H, et al. Results of phase Ш study of paclitaxel in the management of advanced cancer patients. Chin J Clin Pharmacol 1999;15:241-5.
- Zhang F, Fan Q. Paclitaxel combined with nedaplatin in the treatment of advanced esophageal cancer. Chin J Practi Med 2010;37:25-6.
- Dombernowsky P, Giaccone G, Sandler A, et al. Gemcitabine and paclitaxel combinations in non-small cell lung cancer. Semin Oncol 1998;25:44-50. [PubMed]
- Ishikawa T, Shimizu D, Kito A, et al. Breast cancer manifested by hematologic disorders. J Thorac Dis 2012;4:650-4. [PubMed]
- Zhan Y, Chen Y, Liu R, et al. Potentiation of paclitaxel activity by curcumin in human breast cancer cell by modulating apoptosis and inhibiting EGFR signaling. Arch Pharm Res 2013. [Epub ahead of print]. [PubMed]
- Dragowska WH, Weppler SA, Wang JC, et al. Induction of autophagy is an early response to gefitinib and a potential therapeutic target in breast cancer. PLoS One 2013;8:e76503. [PubMed]
- Yu SY, Liu HF, Wang SP, et al. Evidence of securin-mediated resistance to gefitinib-induced apoptosis in human cancer cells. Chem Biol Interact 2013;203:412-22. [PubMed]
- Hussain A, Mohsin J, Prabhu SA, et al. Sulforaphane inhibits growth of human breast cancer cells and augments the therapeutic index of the chemotherapeutic drug, gemcitabine. Asian Pac J Cancer Prev 2013;14:5855-60. [PubMed]
- Kawaguchi Ushio A, Hattori M, Kohno N, et al. Gemcitabine-induced tumor lysis syndrome caused by recurrent breast cancer in a patient without hemodialysis. Gan To Kagaku Ryoho 2013;40:1529-32. [PubMed]
- Kanazawa S, Ogata H, Magoshi S, et al. Remarkable improvement in a patient with metastatic and locally advanced HER2-positive breast cancer treated with trastuzumab plus vinorelbine. Gan To Kagaku Ryoho 2012;39:445-9. [PubMed]


