A phase I study of nimotuzumab plus docetaxel in chemotherapy-refractory/resistant patients with advanced non-small-cell lung cancer
Introduction
Non-small-cell lung cancer (NSCLC) accounts for approximately 85% of all newly diagnosed lung cancer. Unfortunately, most patients with NSCLC already have advanced disease at diagnosis. For these patients, chemotherapy represents the standard of care; however, a plateau has been reached with this approach to date. Improvements in the knowledge of tumor biology and mechanisms of oncogenesis have identified epidermal growth factor receptor (EGFR), a member of the ErbB family, as a molecular target for NSCLC treatment (1). Strategies to block EGFR include tyrosine kinase inhibitors, monoclonal antibodies (mAbs), ligand-linked toxins, and antisense approaches (2-4).
Various mAbs against EGFR have been or are currently being tested in preclinical and clinical trials to determine their efficacy as anticancer agents. Cetuximab, an anti-EGFR immunoglobulin G1 mAb, has shown activity when given in combination with cisplatin in preclinical studies. A randomized phase III trial in advanced NSCLC patients yielded a statistically significant survival advantage for patients treated with cetuximab plus chemotherapy versus chemotherapy alone (5). In addition, a retrospective biomarker analysis of this study showed that greater efficacy was observed the patients with high EGFR expression compared to those with low expression of EGFR (6). Nimotuzumab (also known as h-R3) is a humanized anti-EGFR mAb, which is currently under clinical evaluation. In a preclinical study, nimotuzumab showed marked antiproliferative, proapoptotic, and antiangiogenic effects in tumors that overexpress EGFR (7). In early clinical trials, nimotuzumab exhibited a longer half-life and a greater area under the curve (AUC) plasma concentrations in comparison with other anti-EGFR antibodies (8). A phase I/II trial showed that nimotuzumab was well tolerated and could enhance the curative potential of radiation in patients with advanced head and neck cancer (9). Given that little is known of the antitumor activity of nimotuzumab in NSCLC, we investigated the efficacy and safety of this mAb combined with docetaxel in NSCLC patients with EGFR expression.
Methods
Design of the study
This was a single-center, open-label, dose-escalating phase I trial of nimotuzumab plus chemotherapy in Chinese NSCLC patients. The patients were treated according to a dose escalation schedule. Ethical approval was obtained from Ethics committee of Beijing Cancer hospital. The study was conducted according to the ethical principles laid down in the Declaration of Helsinki, and was consistent with the ethical Good Clinical Practice Guidelines.
The primary objectives were to determine the safety and tolerability of this combined treatment. The secondary objectives were to determine the overall response rate, disease control rate (DCR), and survival time.
Nimotuzumab (200, 400, and 600 mg) was administered weekly from week 1 to week 3 with chemotherapy (docetaxel 75 mg/m2 every 3 weeks). If tumor control was achieved, nimotuzumab treatment was continued every week until unacceptable toxicity or disease progression occurred. Docetaxel was administered intravenously over 1 hour on day 1 of each cycle, with standard dexamethasone premedication. Each cycle was defined as 21 days, and dose escalation was based on safety data from the first cycle of each cohort. Nimotuzumab was supplied by Biotech Pharmaceutical Co. Ltd. in China. Docetaxel is commercially available (Sanofi-Aventis Pharmaceuticals, Inc.)
For phase I study, three patients were enrolled in each dose level. If no dose-limiting toxicities (DLT) were seen during the first 21-day cycle, three patients were enrolled at the next dose level. If one patient experienced DLT, an additional three patients were to be enrolled at that dose level. If two or more of six patients experienced DLT at any dose level, this would be considered the maximum tolerated dose, and the dose levels below would be considered the recommended phase II dose. If no DLT occurred at the highest planned dose level, this would be considered the recommended phase II dose.
Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 3.0. To be considered dose limiting, toxicity had to occur during the first cycle of therapy, and be deemed at least possibly related to protocol treatment. DLT was defined as any of the following: Grade 4 neutropenia lasting more than 7 days; Grade ≥3 febrile neutropenia; platelet count <25×109/L; thrombocytopenia resulting in Grade ≥2 hemorrhage; Grade ≥3 infection with Grade ≥3 neutropenia (<1.0×109/L); Grade 3 or 4 non-hematologic toxicity except alopecia, nausea, emesis, diarrhea (unless persistent despite preventive therapy); and inability to administer the combination therapy on day 1 of cycle 2 within 2 weeks after completing previous cycle.
Patients and eligibility criteria
All patients provided their written informed consent. Patients with histological or cytological confirmed advanced (stage IV) NSCLC and immunohistochemically evidence of positive EGFR expression were eligible for this study. Patients must have had progressive disease after receiving only one prior platinum-based chemotherapy regimen (For the patients who received neoadjuvant chemotherapy or adjuvant chemotherapy, only those surviving disease-free within the past 6 months were eligible to enroll). The last dose of chemotherapy must have been finished at least 3 weeks before the study, and the patients must have recovered from the acute toxicity of chemotherapy. Patients who previously received radiotherapy were eligible for the study. Bone marrow influenced by radiotherapy should have been less than 25% of the total quantity of general bone marrow, and patients could not have received whole pelvis radiation. Previous radiotherapy must have been finished at least 4 weeks before enrollment. Patients must have had at least 1 tumor lesion that could be accurately measured by magnetic resonance imaging (MRI) or computed tomography (CT) in at least one dimension with longest diameter to be recorded as ≥20 mm using conventional techniques or ≥10 mm with spiral CT. Other inclusion criteria included Eastern Cooperative Oncology Group (ECOG) performance status 0–2; life expectancy ≥12 weeks; adequate organ function (bone marrow: absolute neutrophil count ≥1.5×109/L, platelet count ≥100×109/L, hemoglobin ≥90 g/L; liver function: bilirubin (BIL) ≤1.5× upper limit of normal (ULN), alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) ≤2.5× ULN or ≤5 ULN (Liver metastasis); renal function: creatinine clearance rate (CCR) ≥60 mL/min); no history of clinically significant or uncontrolled cardiac disease; and normal electrocardiogram (ECG). In addition, patients of both genders were required to use effective contraceptive methods during and 3 months after the end of the study. Female subjects must not have been breastfeeding, and serum or urine pregnancy test should have been negative. Signed informed consent must have been attained and submitted to the organization of research.
Exclusion criteria included symptomatic brain metastasis; previous treatment including docetaxel, anti-EGFR mAbs, anti-angiogenesis-targeted medicine, and small molecule tyrosine kinase inhibitors (TKIs); receiving other anti-cancer drugs during the study; uncontrolled pleural effusion, seroperitoneum, or pericardial effusion; serious illness or other malignancies diagnosed within past 5 years; any serious active infection; a second primary malignant tumor; serious accompanying disease that would influence the study results (such as cardiac disease, diabetes mellitus, etc.); contraindication of hormone therapy; and previous definable symptomatic peripheral neuropathy.
Adverse events were evaluated using NCI CTCAE, version 3.0. Toxicity assessments were made weekly during the induction period, and then every other week. Treatment response was evaluated after 8 weeks of treatment by CT, and then every 2 months using the Response Evaluation Criteria for Solid Tumors (RECIST) thereafter. DCR (which includes the complete and partial response, plus stable disease) was also assessed. The “response” refers to the best response recorded from the start to the end of the study. Survival analysis was calculated using the Kaplan-Meier estimator.
EGFR detection by immunohistochemistry (IHC)
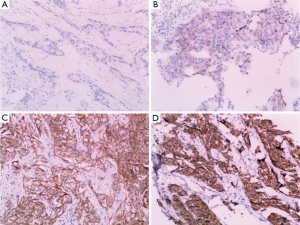
The biopsy specimens were obtained from 12 patients and subjected to an immunochemical assay. The immunoreactivity of EGFR was classified into four groups according to the intensity of cell membrane EGFR staining in the whole tumor: high (+++), markedly stronger staining than normal lung epithelium; medium (++), moderately stronger staining than normal lung epithelium; low (+), staining identical to that of normal epithelium; and negative (−), faint staining.
Statistical analysis
It was estimated that approximately 12 to 18 patients would be required to complete the dose escalation of the study. Descriptive statistics, such as the median, frequency and proportion, were used to summarize the cohort of patients along with 95% confidence intervals (CI) where possible. The Kaplan-Meier and log-rank test were used to estimate progression-free survival (PFS) and survival statistics. Overall survival (OS) was calculated from the date the patient first received study treatment until the date of death or last date the patient was known to be alive. PFS was calculated from the date the patient first received study treatment until the first date of progression. SPSS software, version 11.5 (IBM Corporation, Armonk, NY, USA), was used.
Results
The safety and efficacy of nimotuzumab (h-R3) combined with docetaxel. There were 12 patients with stage IV NSCLC enrolled in this study. In total, they received 37 cycles of chemotherapy and 244 doses of nimotuzumab, with doses ranging from 200 to 600 mg/week. The patient characteristics are summarized in Tables 1 and 2. Among the 12 patients enrolled, 3 patients finished less than two cycles of chemotherapy due to toxicities and disease progression. The longest administration was 79 weeks at the 200 mg/week dose level. There were eight patients (66.7%) that received nimotuzumab for longer than 16 weeks.
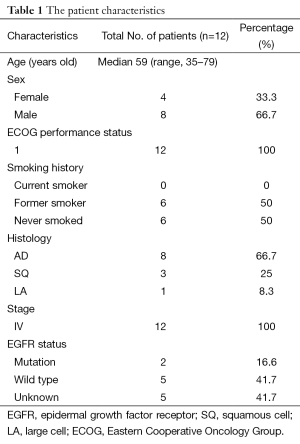
Full table
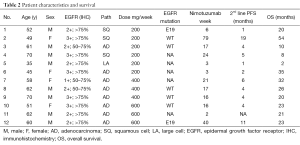
Full table
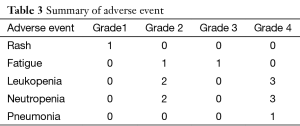
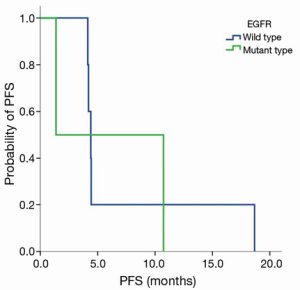
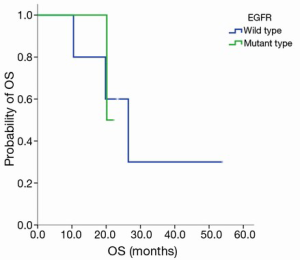
Grade III–IV toxicities (accounting for 50% of all adverse events) included neutropenia and fatigue, and other toxicities included rash, fatigue (Table 3). DLT occurred with Grade 3 fatigue at the 200 mg dose level of nimotuzumab and Grade 4 neutropenia with pneumonia at the 600 mg dose level of nimotuzumab. No objective responses were seen, and stable disease was observed in eight patients (66.7%). The median PFS was 4.4 months in all patients, 1.4 months in patients with the EGFR mutation and 4.4 months in patients with wild type EGFR (EGFR WT), P=0.806 (Figure S1). The median survival time (MST) was 21.1 months in all patients, 21.1 months in patients with EGFR mutation, and 26.4 months in patients with EGFR WT, P=0.981 (Figure S2). Three patients were still alive at the time of data analysis (23+, 23+, and 54+ months).
Full table
EGFR expression and efficacy of nimotuzumab (h-R3) combined with docetaxel
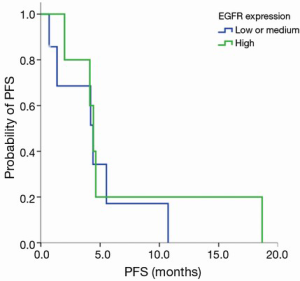
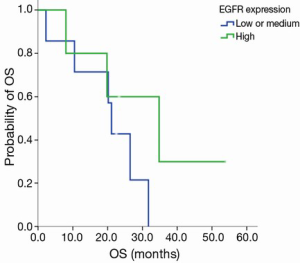
EGFR expression status was determined in 12 patients. The intensity of cell membrane EGFR staining was classified into four groups (Figure 1). The frequency of staining in all of the samples was 50–75%. High expression was observed in five cases, and medium or low expression was observed in seven cases (Table 2). There was no association between EGFR expression intensity and PFS or overall survial due to nimotuzumab-based treatment (P=0.594, P=0.225) (Figure S3,Figure S4).
Discussion
This study demonstrates the tolerability of nimotuzumab combined with chemotherapy in Chinese NSCLC patients. The toxicity profile of the nimotuzumab and docetaxel combination appeared favorable, and complications were mild. Grade III–IV toxicities (accounting for 50% of all adverse events) included neutropenia and fatigue, and other toxicities included rash (occurred in only one patient). DLT occurred with Grade 3 fatigue at the 200 mg dose level of nimotuzumab and Grade 4 neutropenia with pneumonia at the 600 mg dose level of nimotuzumab. Most of toxicities were due to docetaxel, as reported in other studies (10-12). The toxicity and safety of nimotuzumab have been assessed in several pre-clinical and clinical studies, and it is noteworthy that the side effects usually caused by EGFR inhibitors, especially rashes and other skin toxicities, were negligible (13). Scientists have hypothesized that this is because nimotuzumab binds only to cells that express moderate to high levels of EGFR.
Nimotuzumab attaches to EGFR with moderate binding affinity compared with cetuximab, which has a greater than 10-fold higher binding affinity (14). In vitro studies have shown that nimotuzumab binds bivalently (i.e., with both antibody arms to two targets simultaneously) to EGFR with a moderate or high density, which is a stable pattern of attachment (15). In normal tissues with low EGFR density, nimotuzumab has less affinity and binds to EGFR with less avidity, which spares the normal tissues, including skin and mucosa, from severe cytotoxicity. This explains why treatment with nimotuzumab leads to less treatment-related toxicities in clinical applications, while displaying similar or even superior anticancer effects as compared to other anti-EGFR mAbs.
There was a phase II study compared nimotuzumab plus chemotherapy or chemotherapy alone in first line of NSCLC. The objective response rate was significantly higher in the nimotuzumab group than in the control group in the intent-to-treat population (54% vs. 34.5%; P=0.04). No significant differences in PFS and OS were observed. Safety profiles were comparable between the two groups (16). In our study, the combination did not yield objective responses in pre-treated NSCLC patients; stable disease was observed in eight patients (66.7%). This result is consistent with the published literature in the setting of second-line single-agent docetaxel. The lack of objective responses is also consistent with docetaxel therapy, with a historical response rate of only 6% to 9% in the randomized trials of second-line treatment in NSCLC (2,10-12). The median PFS time of combination therapy was 4.4 months, which was better than the previous results of second-line single-agent docetaxel (10-12). Despite the small number of patients recruited and the heterogeneity of disease stages, the results of the nimotuzumab and chemotherapy combination are promising enough to be worthy of further investigation.
It has been shown that some clinical and molecular predictive markers can be used for sensitive selection of NSCLC for EGFR tyrosine kinase inhibitors (EGFR-TKIs), such as adenocarcinoma (AD) histology, the female gender, no history of smoking, East Asian ethnicity, and the presence of EGFR mutations (17). EGFR mAbs, which target the extracellular domain of the receptor, have effects that differ from those of EGFR TKIs. Therefore, the responsiveness to nimotuzumab may be related to the level of EGFR expression rather than to the EGFR mutation. In addition, K-RAS mutations have been identified as predictors of non-responsiveness to EGFR-targeting agents in colon cancer (18). In this study, we could not draw any definitive conclusions because only 12 patients underwent biomarker analysis. Further studies are required to identify the molecular determinants that can influence the activity of nimotuzumab.
Conclusions
In conclusion, nimotuzumab and docetaxel combination therapy was found to be well tolerated and efficacious. Future clinical investigation of nimotuzumab is warranted in NSCLC patients.
Acknowledgements
We thank all the physicians, nurses, and patients who participated in this study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Baselga J. Why the epidermal growth factor receptor? The rationale for cancer therapy. Oncologist 2002;7 Suppl 4:2-8. [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [PubMed]
- Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 2008;372:1809-18. [PubMed]
- Butts CA, Bodkin D, Middleman EL, et al. Randomized phase II study of gemcitabine plus cisplatin or carboplatin [corrected], with or without cetuximab, as first-line therapy for patients with advanced or metastatic non small-cell lung cancer. J Clin Oncol 2007;25:5777-84. [PubMed]
- Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009;373:1525-31. [PubMed]
- Douillard JY, Pirker R, O'Byrne KJ, et al. Relationship between EGFR expression, EGFR mutation status, and the efficacy of chemotherapy plus cetuximab in FLEX study patients with advanced non-small-cell lung cancer. J Thorac Oncol 2014;9:717-24. [PubMed]
- Crombet-Ramos T, Rak J, Pérez R, et al. Antiproliferative, antiangiogenic and proapoptotic activity of h-R3: A humanized anti-EGFR antibody. Int J Cancer 2002;101:567-75. [PubMed]
- Crombet T, Torres L, Neninger E, et al. Pharmacological evaluation of humanized anti-epidermal growth factor receptor, monoclonal antibody h-R3, in patients with advanced epithelial-derived cancer. J Immunother 2003;26:139-48. [PubMed]
- Crombet T, Osorio M, Cruz T, et al. Use of the humanized anti-epidermal growth factor receptor monoclonal antibody h-R3 in combination with radiotherapy in the treatment of locally advanced head and neck cancer patients. J Clin Oncol 2004;22:1646-54. [PubMed]
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000;18:2095-103. [PubMed]
- Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol 2000;18:2354-62. [PubMed]
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97. [PubMed]
- Boland W, Bebb G. The emerging role of nimotuzumab in the treatment of non-small cell lung cancer. Biologics 2010;4:289-98. [PubMed]
- Garrido G, Tikhomirov IA, Rabasa A, et al. Bivalent binding by intermediate affinity of nimotuzumab: a contribution to explain antibody clinical profile. Cancer Biol Ther 2011;11:373-82. [PubMed]
- Ramakrishnan MS, Eswaraiah A, Crombet T, et al. Nimotuzumab, a promising therapeutic monoclonal for treatment of tumors of epithelial origin. MAbs 2009;1:41-8. [PubMed]
- Babu KG, Prabhash K, Vaid AK, et al. Nimotuzumab plus chemotherapy versus chemotherapy alone in advanced non-small-cell lung cancer: a multicenter, randomized, open-label Phase II study. Onco Targets Ther 2014;7:1051-60. [PubMed]
- Ho C, Murray N, Laskin J, et al. Asian ethnicity and adenocarcinoma histology continues to predict response to gefitinib in patients treated for advanced non-small cell carcinoma of the lung in North America. Lung Cancer 2005;49:225-31. [PubMed]
- Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008;359:1757-65. [PubMed]


