Immune responses of dendritic cells combined with tumor-derived autophagosome vaccine on hepatocellular carcinoma
Introduction
Liver cancer is one of the most common malignancies in China with 358,840 incident cases and 312,432 deaths in 2010 (1). In 2012, half of all new cases of liver cancer and related deaths worldwide were estimated to occur in China (2). Hepatocellular carcinoma (HCC) accounts for 70–80% of all liver cancers. The clinical evidence shows that the majority of liver cancer patients have poor responses to chemotherapy and radiotherapy. Therefore, new methods and means are needed for the clinical treatment of liver cancer.
Cross-presentation is the ability of host professional antigen-presenting cells (APCs) to capture, process, and present exogenous antigens to T cells (3). Antigen cross-presentation is pivotal for the initiation of T-cell immune responses, and is necessary for the elimination of many pathogens (4,5). Evidence in the literature suggests that cross-presentation of melanoma antigens during vaccination was essential for an effective anti-tumor therapy. These data indicate that targeting cross-presentation may be a unique approach for immunotherapy for cancer (6). Therefore, the first critical component of successful therapeutic cancer vaccines is to maximize the efficiency of cross-presentation (7).
Autophagy is a basic cellular process in which unnecessary or dysfunctional cellular components are sequestered by autophagosomes and delivered to lysosomes for degradation (8,9). As we know, autophagy in tumor-targeted therapy is thought to be a “double-edged sword”, which plays a prodeath or prosurvival role (10-12). On the other hand, it has been reported that autophagy can be induced by metabolic stress and antitumor therapies (13,14). Although the role of autophagy in tumor therapy is complicated and not yet fully understood, it has been considered an attractive approach for anticancer therapy.
Accumulating evidences suggest that autophagy plays an important role in both innate and adaptive immunity (15,16). Moreover, it has been shown that autophagy in tumor cells is essential for cross-presentation of tumor antigens and subsequent induction of tumor immunity (17). As we know, proteins in tumor cells are degraded either by the autophagy-lysosome pathway or the ubiquitin-proteasome system. Though little about the transition between the two pathways is known, it is believed that short-lived proteins, including defective ribosomal products (DRiPs) are ubiquitinated and degraded by proteasomes and long-lived proteins are separated into autophagosomes for lysosomal degradation. Autophagosomes have been identified as critical tumor-antigen carriers for cross-presentation (18). Recently, some results showed the enhanced antigen presentation was related to autophagy (19,20). In this study, autophagosomes from SMMC7721, a human HCC cell line, were induced by rapamycin (autophagy revulsive), bortezomib (proteasome inhibitor), and ammonium chloride (lysosomotropic agent), by which the fusion of lysosomes and phagosomes would be prevented according to previous reports (18,21).
Autophagosomes of tumor cells were isolated from both the cells and the culture medium, and these DRiPs containing autophagosome-rich blebs were termed “DRibbles”. Recently, it has been reported that DRibbles act as a potent antigen source and induce enhanced immune responses in vaccine studies (21). Our present study was to induce and characterize DRibbles from the SMMC7721 cell line, and further to elucidate its possible therapeutic antitumor efficacy on HCC.
Materials and methods
Patients
In this study, 17 HCC patients, who were treated in Department of Hepatobiliary Surgery, the Second Hospital of Nanjing from Feb 2011 to Feb 2012, were included. Inclusion criteria included primary liver cancer and Eastern Cooperative Oncology Group (ECOG) score 0-3. Exclusion criteria included pregnancy, active liver disease, or metastatic liver cancer. The study was approved by the Ethics Committee of the Second Hospital of Nanjing, and conducted in accordance with the ethical standards described in the Helsinki Declaration (revised in 1983), using Good Clinical Practices (GCP) after approval by the hospital. All patients were gave written informed consent prior to participation. Key eligibility criteria included previously untreated HCC that was not considered curable by resection or liver transplant.
Culture of DCs and SMMC7721 cell line
CS-3000 blood cell separator was used to collect peripheral blood mononuclear cell (PBMC) of HCC patients. PBMCs (1×106/well) were cultured in RPMI 1640 complete medium containing 10% fetal bovine serum (FBS; Invitrogen, Grand Island, NY, USA), 10 ng/mL murine granulocyte-macrophage colony-stimulating factor (GM-CSF; eBioscience, San Diego, CA, USA), and 1 ng/mL murine interleukin-4 (IL-4; eBioscience) for 5 d. Half the medium with GM-CSF and IL-4 was gently replaced on d 2 and d 4. SMMC7721 human HCC cell line was cultured in DMEM supplemented with 10% FBS, 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin (Invitrogen, Grand Island, NY, USA).
Preparation of DRibbles
SMMC7721 cells were cultured in DMEM complete medium containing 10% FBS, and treated with rapamycin (Enzo Life Sciences), bortezomib (Velcade, Millennium Pharmaceuticals) and NH4Cl for 16–24 h. The resulting suspension was pre-cleared by centrifugation at 1,600 r/min for 10 min, and the supernantant was then separated from the crude autophagosome-containing large vesicles (DRibbles) consisting of cytosolic components by a 30-min centrifugation at 12,000 r/min followed by washing with phosphate-buffered saline (PBS) and a second 30 min centrifugation at 12,000 r/min. The DRibbles were then aliquoted into tubes for freezing. The total protein concentration was measured by bicinchoninic acid (BCA) assay according to the manufacturer’s protocol (BCA Protein Assay Kit, Thermo). DRibbles were resuspended by PBS with the total concentration of 1 mg/mL and kept at −20 °C for short-term storage (less than 1 month) or −80 °C for long-term storage.
Treatment
The blood was sampled on the first day. On the 5th day, before injection, DRibble vaccine was prepared under 10 Gy γ-ray irradiation for 30 min to remove non-specific antigen. Then the patients were vaccinated with DCs (1×106) loaded with DRibbles (25 µg/mL) in a total volume of 40 µL PBS by B ultrasound-guided percutaneous injection of bilateral inguinal lymph node. On the 8th day, the second treatment circle began, and generally 4–5 times of 7-day treatment were given as a course. After treatment, the patients’ blood was used for immunological detection by the following methods. The patients were followed up for an average of 25.4 months (range, 12–48 months), and 1, 2 and 3-year survival was calculated.
Flow cytometry
For analysis of surface markers, DCs were incubated with DRibbles (0, 5, 25 and 50 µg/mL) for 24 h (treated with PBS as a control). Then, cells were harvested, thoroughly washed, and incubated with fluorochrome-conjugated specific antibodies for CD80, CD83, CD86 and HLA-DR (eBioscience) at recommended dilutions for 30 min at 4 °C. Meanwhile, mononuclear cells obtained from lymph nodes and spleens were loaded with DRibbles (25 g/mL) for 72 h and incubated with fluorochrome-conjugated specific antibodies (CD80-PE, CD83-APC, CD86-FITC, HLA-DR-perCp, eBioscience) under the same conditions.
Analysis of total number of T cells
After collecting 5 mL peripheral blood of patients and centrifuging at 1,000 r/min for 8 min, the cells was washed with 5 mL PBS and centrifuged to remove serum specific antigens, and then suspended in PBS to 1×106 cells/mL. Then 20 µL of mouse antihuman monoclonal antibodies against CD3-PE, CD4-APC, CD8-FITC and CD56-perCp were added to 1,000 µL cell suspension, and labeled at 4 °C for 30 min. After centrifuging at 1,000 r/min for 5 min, the cells were washed with 3 mL PBS twice and finally suspended in PBS to 1×106 cells/mL. Flow cytometry was used to detect the phenotype.
Immunization and detection of immune responses
DCs (20 µL samples containing 106 cells) pulsed by SMMC7721-derived DRibbles (20 µg total protein) and the same volume of PBS were injected directly into both inguinal lymph nodes of patient on d 1, and were boosted by 100 µL DCs-DRibbles or PBS subcutaneously on d 2, d 3 and d 7. PBMC were collected, red cells were lysed by Red Cell Lyse Buffer, and lymphocytes were resuspended in RPMI 1640 complete medium and seeded into 24-well plates. SMMC7721-derived DRibbles (30, 10, 3, and 0 µg total protein/mL), inactivated SMMC7721 cells and SMMC7721 cell lysates (30 µg total protein/mL) were added to the cell cultures. After 72 h of incubation, the cell culture supernatants were collected, and the concentration of IFN-γ was measured by enzyme-linked immunosorbnent assay (ELISA) (eBioscience). The lymphocytes were selected using CD8+ cells and CD4+ cells on magnetic beads (Dynabeads FlowComp Mouse CD8, Invitrogen) and co-cultured with different antigens. IFN-γ was measured after 72 h of co-incubation.
Statistical analysis
All data were expressed as 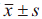 , and T cell counts before and after the injection of DCs-DRibbles vaccine were analyzed by paired t-test using SPSS 19.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
, and T cell counts before and after the injection of DCs-DRibbles vaccine were analyzed by paired t-test using SPSS 19.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
Results
Patient characteristics
Among the enrolled patients, 9 (52.9%) were males, and the median age was 56 years old [interquartile range (IQR), 45–64 years]. At diagnosis, 10 (58.8%) were classified into Child-Pugh class A, 6 (35.3%) into class B, and 1 (5.9%) into class C. There were 4 (23.5%) patients at TNM stage I, 6 (35.3%) at stage II, 6 (35.3%) at stage III, and 1 (5.9%) at stage IV. The median tumor size was 6.2 cm (IQR, 2.5–19.5 cm), and the number of patients with solitary tumor was 11 (64.7%). The case numbers of presence of portal vein thrombosis (PVT) and extra-hepatic metastasis were 5 (29.4%) and 3 (17.6%), respectively.
Morphology of DC cells
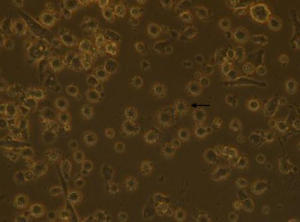
Adherent cells were obtained after 3 h. After 3–4 d of cell factor culture, the cell morphology was irregular. With the extension of time, the suspended cells increased gradually, neurites extended from the cells, and cells showed the trend of cluster formation. As shown in Figure 1, there are many short and small burrs on the surface of DCs.
DRibbles-induced expression of surface molecules on DCs
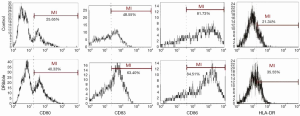
To investigate the role of DRibbles in modulating DC function, we evaluated the effects of DRibbles on the expressions of CD80, CD83, CD86 and HLA-DR of DCs. As shown in Figure 2, DRibbles significantly induced the expression of CD80, CD83, CD86 and HLA-DR of DCs.
Analysis of total number of T cells
CD4+ T cell increased significantly after injection of DCs-DRibbles vaccine compared with before (t=2.18, P<0.05) (Table 1).
Immune responses of lymphocytes induced by DCs-DRibbles
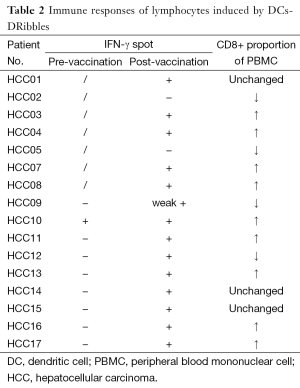
IFN-γ was detected in the supernatant after 72 h of incubation. The ELISA showed that IFN-γ post vaccination increased than before. Higher levels of IFN-γ were detected in the CD8+ cells than in CD4+ T cells. These results suggested that DCs-DRibbles vaccine could induce antigen-specific cellular immune response on HCC and could prime strong CD8+ T cell responses, supporting it as a tumor vaccine candidate. CD8+ proportion of PBMC increased in 9 patients. IFN-γ spot increased in 14 patients post vaccination (Table 2).
Full table
Survival rate
The 1-, 2- and 3-year survival was 17/17, 16/17 and 12/17, respectively. Eight patients achieved partial remission (PR), 5 stable disease (SD), and 4 progressive disease (PD).
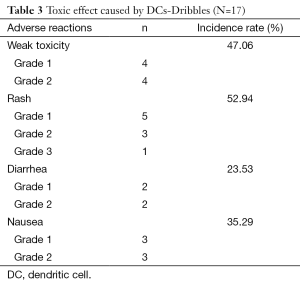
Safety assessment
The majority of patients had grade 1–2 toxicity (Table 3) and only one patient had grade 3 toxicity, indicating the vaccine was safe.
Full table
Discussion
HCC is one of the most deadly cancers in the world, ranking third among all cancer-related deaths. Potentially curative therapies can be offered to approximately 30% of patients, and are complicated by a high rate of recurrence (22). Although many kinds of antitumor strategies have been developed, HCC is still treated traditionally with surgery, radiotherapy and chemotherapy (23). The limitations in treatment efficacy of current therapeutic modalities point to the urgent need of new efficient therapeutic strategies to decrease the incidence and mortality of HCC. There is thus an urgent need of multiple, successive treatment options for HCC, including immunotherapy. In a previous study, we documented the unique characteristics and potent antitumor efficacy of an autophagosome-based DRibble vaccine (24).
In past years, a variety of cancer vaccines have been used in immunotherapy trials (25,26). These vaccines highlight new therapeutic approaches to enhance the patients’ own immunity. Therefore, tumor-antigen inclusion and efficient cross-presentation are important elements for successful treatment with cancer vaccines (25). In this study, we demonstrated that DRibbles deriving from the SMMC-7721 cell line could enhance co-stimulatory molecule expression in DCs and B cells and strengthen the immunostimulatory function of DCs, and explored the efficacy of DRibbles in clinical trials.
In this study, we focused on DRibble modulation on APCs, especially on DCs. The features of DCs shown in Figure 1 were in accordance with autophagosomes in previous research (25,26). Our study showed that DRibbles could induce expression of CD80, CD83, CD86 and HLA-DR on DCs (Figure 2). These co-stimulatory molecules were upregulated to engage in the APC-T cell interaction, which were key factors for the activity of APCs and subsequent immune responses. This study also found a trend toward higher production of IFN-γ by CD8+ T cells from vaccinated patients, indicating the priming of tumor antigen-specific cytotoxic T lymphocyte (CTL) responses by this novel form of antigen (27).
Conclusions
We identified the unique characteristics and potential antitumor efficacy of DRibbles, and provided insights into the mechanism of DRibbles which could be responsible for their efficacy as a novel cancer immunotherapy. The efficacy of DRibbles should be further determined in future clinical trials.
Acknowledgements
Funding: This study was supported by Nanjing Medical Science and Technique Development Foundation, Nanjing Department of Health (Grant: QRX11235 and Grant: ZDX12008), and Jiangsu Science and Technology Project of Clinical Medicine Foundation, Science and Technology Department of Jiangsu Province (BL2014005).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chen W, Zheng R, Zhang S, et al. Annual report on status of cancer in China, 2010. Chin J Cancer Res 2014;26:48-58. [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [PubMed]
- Bevan MJ. Cross-priming. Nat Immunol 2006;7:363-5. [PubMed]
- Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. 1976. J Immunol 2010;185:1361-6. [PubMed]
- Fu C, Liang X, Cui W, et al. β-Catenin in dendritic cells exerts opposite functions in cross-priming and maintenance of CD8+ T cells through regulation of IL-10. Proc Natl Acad Sci U S A 2015;112:2823-8. [PubMed]
- Lazzaro S, Giovani C, Mangiavacchi S, et al. CD8 T-cell priming upon mRNA vaccination is restricted to bone-marrow-derived antigen-presenting cells and may involve antigen transfer from myocytes. Immunology 2015;146:312-26. [PubMed]
- Burgdorf S, Kurts C. Endocytosis mechanisms and the cell biology of antigen presentation. Curr Opin Immunol 2008;20:89-95. [PubMed]
- Lin NY, Beyer C, Giessl A, et al. Autophagy regulates TNFα-mediated joint destruction in experimental arthritis. Ann Rheum Dis 2013;72:761-8. [PubMed]
- Bejarano E, Yuste A, Patel B, et al. Connexins modulate autophagosome biogenesis. Nat Cell Biol 2014;16:401-14. [PubMed]
- Dikic I, Johansen T, Kirkin V. Selective autophagy in cancer development and therapy. Cancer Res 2010;70:3431-4. [PubMed]
- Ojha R, Bhattacharyya S, Singh SK. Autophagy in Cancer Stem Cells: A Potential Link Between Chemoresistance, Recurrence, and Metastasis. Biores Open Access 2015;4:97-108. [PubMed]
- Levy JM, Thorburn A. Targeting autophagy during cancer therapy to improve clinical outcomes. Pharmacol Ther 2011;131:130-41. [PubMed]
- Amaravadi RK, Lippincott-Schwartz J, Yin XM, et al. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res 2011;17:654-66. [PubMed]
- Liu EY, Ryan KM. Autophagy and cancer – issues we need to digest. J Cell Sci 2012;125:2349-58. [PubMed]
- Shibutani ST, Saitoh T, Nowag H, et al. Autophagy and autophagy-related proteins in the immune system. Nat Immunol 2015;16:1014-24. [PubMed]
- Heath RJ, Xavier RJ. Autophagy, immunity and human disease. Curr Opin Gastroenterol 2009;25:512-20. [PubMed]
- Li Y, Wang LX, Yang G, et al. Efficient cross-presentation depends on autophagy in tumor cells. Cancer Res 2008;68:6889-95. [PubMed]
- Koukourakis MI, Kalamida D, Giatromanolaki A, et al. Autophagosome Proteins LC3A, LC3B and LC3C Have Distinct Subcellular Distribution Kinetics and Expression in Cancer Cell Lines. PLoS One 2015;10:e0137675. [PubMed]
- English L, Chemali M, Duron J, et al. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat Immunol 2009;10:480-7. [PubMed]
- Saiga H, Nieuwenhuizen N, Gengenbacher M, et al. The Recombinant BCG ΔureC::hly Vaccine Targets the AIM2 Inflammasome to Induce Autophagy and Inflammation. J Infect Dis 2015;211:1831-41. [PubMed]
- Twitty CG, Jensen SM, Hu HM, et al. Tumor-derived autophagosome vaccine: induction of cross-protective immune responses against short-lived proteins through a p62-dependent mechanism. Clin Cancer Res 2011;17:6467-81. [PubMed]
- Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer 2006;6:674-87. [PubMed]
- Freiser ME, Serafini P, Weed DT. The immune system and head and neck squamous cell carcinoma: from carcinogenesis to new therapeutic opportunities. Immunol Res 2013;57:52-69. [PubMed]
- Yi YX, Zhao W, Wu YW, et al. The establishment of hepatocellular carcinoma model in mice and the biological treatment effect. Zhong Guo Ji Ceng Yi Yao 2013;20:3212-4. (in Chinese).
- Li Y, Wang LX, Pang P, et al. Tumor-derived autophagosome vaccine: mechanism of cross-presentation and therapeutic efficacy. Clin Cancer Res 2011;17:7047-57. [PubMed]
- Nault JC. Next generation sequencing, inter-tumor heterogeneity and prognosis of hepatitis B related hepatocellular carcinoma. Chin J Cancer Res 2014;26:730-1. [PubMed]
- Qin S, Ma S, Huang X, et al. Th22 cells are associated with hepatocellular carcinoma development and progression. Chin J Cancer Res 2014;26:135-41. [PubMed]