Outcomes of robotic surgery for pancreatic ductal adenocarcinoma
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most malignant digestive tumors due to its high potentials of local invasion and distant metastasis. Surgical resection is the only hope for achieving long-term survival in PDAC patients. Robot-assisted surgery is a novel minimally invasive technique that has been applied in the radical treatment of pancreatic diseases. For the highly malignant PDAC, few studies have explored its role due to the large surgical challenges, high technical requirements, and difficulties in the radical treatment for this malignancy (1-4). In our center, a total of 72 PDAC patients underwent the radical resection using the robotic surgical system between April 2010 and December 2014, and the related data are summarized as follows.
Patients and methods
Clinical data
Seventy-two patients entered the final analysis, among whom there were 49 men and 23 women, with an average age of 62.3±6.5 years. The clinical manifestations included left upper abdominal pain (n=22), jaundice (n=8), progressive emaciation (n=4), and fatigue and anorexia (n=5). The remaining patients had no obvious symptom. The pre-operative imaging detected lesions in the head (n=39; including 3 in the uncinate process), body (n=13), or tail of the pancreas (n=20). The pre-operative CA199 level increased in 52 patients (175.9-4,065.8 U/L; mean: 2,259.3 U/L). Seventeen patients also had diabetes and 18 were accompanied with hypertension. Two patients had a previous history of pancreatitis, and two patients had heart disease (premature ventricular beat and right bundle branch block, respectively). All patients signed the informed consent before the surgery.
The case selection criteria were as follows: the general condition was good and without a history of severe heart and/or lung diseases; could tolerate the general anesthesia; the lesion was solitary and isolated; no local infiltration or distant metastasis was detected on medical imaging; and, there was no history of abdominal operation. Since Buchs et al. (5) had documented that age was not a contraindication of robot-assisted pancreatic surgery, no specific age limits were set in our series. The exclusion criteria were as follows: with any contraindication of open pancreatic surgeries; could not tolerate the pneumoperitoneum; with tight abdominal adhesions; the tumor was too large and had invaded the adjacent organs and large blood vessels, which prevented the laparoscopic operations; and pre-operatively judged as in the American Joint Committee on Cancer (AJCC) stage III or IV.
Surgical techniques
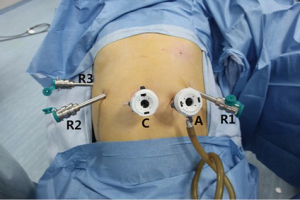
The operation was performed using the da Vinci Model S Surgical System (Intuitive Surgical, Sunnyvale, CA, USA). After tracheal intubation under general anesthesia, the robot operating arms were installed over the patient’s head. The patient’s body position was determined based on the specific surgical procedures. A carbon dioxide (CO2) pneumoperitoneum up to 15 mmHg pressure was established. The laparoscope was inserted to explore the abdominal cavity to further identify whether there was any surgical contraindication. If there was no tight abdominal adhesion or diffuse tumor invasion/metastasis, the surgical instruments were then inserted using the 5-port method (Figure 1). The operating arms were further installed. The intra-operative lymph node dissection was performed according to the 5th criteria published by Japan Pancreas Society in 2002. A specimen bag was inserted via the puncture port to harvest the specimen. If the specimen was too large, the puncture port might be properly enlarged. One or two abdominal drainage tubes were placed after the surgery.
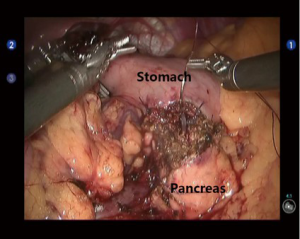
Pancreatoduodenectomy (PD)
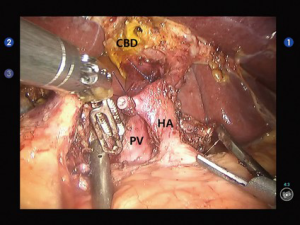
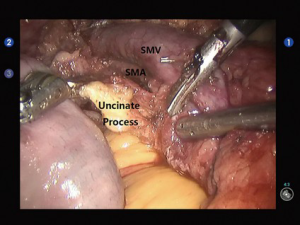
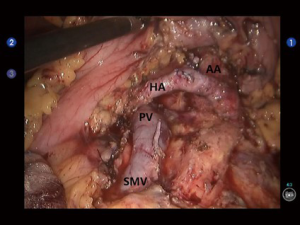
Open the gastrocolic ligament to enter the lesser sac. Isolate the hepatic flexure colon to expose the duodenum and superior mesenteric vein (SMV) for establishing the Kocher incision. Thoroughly isolate the duodenum and pancreatic uncinate, and meanwhile dissect the lymph node stations 13 and 17. Dissect the hepatoduodenal ligament and then the gallbladder; then, the common bile duct was transected at the cystic duct level, and the surrounding lymph nodes were dissected. Dissect the hepatic artery, isolate and ligate the gastroduodenal artery, and dissect the lymph nodes around the hepatic artery and near the portal vein (Figure 2). After the pancreas was divided using the ultrasonic scalpel, the uncinate was isolated upwards along the SMV and the jejunum was divided under the Treitz ligament using the Endo-GIA (60-3.5 mm; Johnson & Johnson, USA) (Figure 3). After the dissection of the lymph node stations 8 and 9, the main pancreatic duct was found at the pancreatic stump and then the supporting tube was placed. Pancreaticojejunostomy, hepaticojejunostomy and gastrojejunostomy were performed using the robotic devices. The specimen was divided and harvested. Double-channel drainage tubes were placed near the pancreaticoenteric and biliary-enteric anastomoses.
Middle pancreatectomy (MP)
Open the gastrocolic ligament to expose the middle section of pancreas. After the tumor was localized, a tunnel behind the pancreas was created by dissecting the upper and lower edges of the pancreas. At the head of the pancreas 1cm away from the tumor, the pancreas was transected using the ultrasonic scalpel or Endo-GIA (60-2.5 mm; Johnson & Johnson, USA). The pancreatic remnant was sutured using the 4-0 Vicryl sutures to prevent any pancreatic fistula. At the tail of the pancreas 2 cm away from the tumor, the pancreas was transected with an ultrasonic scalpel or electrocautery hook. After the main pancreatic duct was found, a proper pancreatic duct-supporting tube was placed. A pancreatic duct-to-gastric mucosa anastomosis was created at the posterior gastric wall near the greater curvature of stomach using the 4-0 Vicryl sutures (interrupted sutures for gastric mucosa, with interrupted fixation at the outer ring) (Figure 4). Double-channel drainage tubes were placed near the pancreaticogastric anastomosis and pancreatic stump (at the head of pancreas).
Distal pancreatectomy (DP)
Open the gastrocolic ligament using the ultrasonic scalpel to expose the pancreas. Dissect the upper and lower edges of the normal pancreatic tissue at the right side of the mass to establish a tunnel behind the pancreas. Then, hang upward the pancreatic tissue to expand the post-pancreatic tunnel. The pancreas was divided about 2 cm away from the right side of the mass using the Endo-GIA (60-2.5 mm; Johnson & Johnson, USA), followed by the clamping of the splenic artery and vein using two hem-o-lok clips. The post-pancreatic gap was dissected leftwards to dissociate the pancreatic tail, splenic artery and vein, and spleen; meanwhile, the lymph node stations 10, 11, and 18 were also dissected. After the specimen was isolated, the lymph node stations 9, 8a, 14, and 16 were dissected; then, the specimen was harvested. After the abdominal cavity was rinsed, the double- and single-channel drainage tubes were placed at the residual pancreas and splenic fossa, respectively (Figure 5).
Results
There were three patients converted to conventional laparotomy. Of these three patients, two were scheduled for robotic PD. One patient, however, was converted to laparotomy because the tumor was too large and the tissue isolation was extremely difficult. In another patient, PD + SMA reconstruction were performed because the tumor invaded the SMA. In the remaining patient who was scheduled for robotic DP, conversion was performed due to the tumor invasion of the transverse colon, left adrenal gland and stomach wall. Robot-assisted surgery in the radical resection of PDAC was successfully performed in the remaining 69 patients, including 37 cases of PD, 2 cases of MP, and 30 cases of DP. In one patient who received DP, the intra-operative exploration showed that the tumor had invaded the SMV; then, reconstruction of the SMV was performed under the robotic devices.
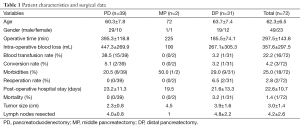
Complications were observed in 18 patients (25.0%), which included pancreatic fistula (n=11), bile leak (n=5), anastomotic bleeding (n=2), pancreatic fistula complicated with portal vein thrombosis (n=1), and anastomotic bleeding complicated with acute renal failure (n=1). Except that one patient was died of postoperative anastomotic bleeding accompanied with acute renal failure and one patient further underwent DSA embolization after anastomotic bleeding, all the remaining patients were smoothly discharged after conservative treatment (Table 1).
Full table
These 72 patients were followed up for 1-45 months (mean, 15.6±5.8 months), with a median overall survival (OS) of 19.6 months. Nineteen patients received routine chemotherapy after the surgery. Fifty-one of the 72 patients are currently alive and well without recurrence. Ten patients died. Eleven patients suffered from recurrence or metastasis, among which 6 had local recurrence, 4 had liver metastasis, and 1 had ascites accompanied with incision site tumor metastasis. The disease-free survival (DFS) was 3-18 months (median, 9.6 months).
Discussion
The pancreas has a complex anatomical structure where the organ is located deeply and has tight connections with the surrounding vessels, making the surgery particularly difficult. Even worse, the PDAC has increased local invasion and distant metastasis in its early stages, posing huge challenge to the surgeons. Thus, the minimally invasive surgery for pancreatic cancer develops slowly. There were few reports on the laparoscopic surgery for pancreatic cancer, which were often accompanied with high rate of conversion to open surgery and high incidence of pancreatic fistula (6-8). The past decade has witnessed the increasingly wider application of robot-assisted laparoscopic surgery (9). Along with the technical development, the robot-assisted surgery for pancreatic diseases has attracted more attention (9). In 2003, Giulianotti et al. completed the first robot-assisted laparoscopic pancreatic resection (10). Since then, more investigations have been made on the applications of various surgical resection procedures for pancreas using the robotic surgical systems (11-13).
Today, the main controversies on the role of minimally invasive surgery for pancreatic malignancies include the effectiveness of radical resection of tumor and the real benefit of patients from the surgery. Therefore, there are even higher requirements on the surgical techniques, which include: (I) the pancreatic cancer is highly malignant and can easily penetrate the pancreatic capsule and thus invade the surrounding tissues/organs, making the resection of the mass particularly difficult; (II) lymphatic metastasis is common in patients with pancreatic cancer, which decides the scope and extent of lymph node dissection and has a fundamental impact on the outcomes of the radical treatment for pancreatic cancer; and (III) for pancreatic cancer patients with portal vein and/or SMV involvement, vascular reconstruction may be required to ensure a good therapeutic effectiveness.
Compared with the conventional laparoscopic surgeries, the robot-assisted surgeries have many advantages such as smaller incisions, faster post-operative recovery, and less trauma and stress response; also, the robotic system can display clear 3-D images, provide Endo WristTM wrist-simulating devices with seven degrees of freedom, and enable tremor filtration, which provides better solutions for the division of pancreas uncinate process, fine separation between pancreatic mass and surrounding tissues, retroperitoneal lymph node dissection, skeletalization of the structures inside the hepatoduodenal ligament, and reconstruction of major vessles (e.g., portal vein). (I) When the robotic surgical system is applied for the radical resection of pancreatic cancer, an upward visual field may be adopted, via which the fine dissection of the tissues at the pancreatic head and neck can be performed at the vessel-free zone among the lower edge of the duodenum, the upper edge of pancreas, the SMV, and the neck of pancreas under direct vision, so as to create a portal vein-pancreas tunnel (14). Thus, the resection of the mass at the pancreatic head/neck can achieve the meeting of the upward and downward operations, which is particularly useful in the division of the uncinate process when a tumor in the uncinate process compresses the vessels; (II) when the robotic system is applied for PD, MP, and DP for the patients with pancreatic cancer, the operator can observe upwardly the post-pancreatic tunnel created during mass resection; meanwhile, the lymph nodes behind the pancreas can be clearly visible in the surgical field. Thus, it increases the completeness of lymph node dissection and increases the rate of R0 resection (compared with the laparoscopic surgeries) (15). Compared with the laparoscopic radical resection of pancreatic cancer, the robotic surgical system enables a finer lymph node dissection, which lowers the risk of vascular injury and uncontrolable bleeding; in particular, it improves the safety during the removal of lymph node stations 7, 8, 9, 11, 12, 14, and 16. In our current study, the lymph nodes were dissected according to the standard radical treatment procedure for pancreatic cancer, during which fewer lymph nodes were removed, which might be explained by the fact that lymph node dissection was not performed after the en bloc resection of the specimen; (III) also in our study, intra-operative exploration in one patient with cancer in the pancreatic head and body showed that the SMV had been invaded by the tumor. After the reconstruction of the SMV, the patient recovered well, without obvious complication. No tumor recurrence or metastasis was noted during the 20-month follow-up. These findings demonstrated the feasibility of robotic surgical system in combination with vascular resection & reconstruction in treating pancreatic cancer; (IV) under the help of the upward operation angle and the flexible Endo WristTM wrist devices in the da Vinci system, the operator can complete the reconstruction of gastrointestinal tract (e.g., pancreaticojejunostomy, hepaticojejunostomy and gastrojejunostomy), which is particularly useful for the minimally invasive surgery for carcinoma in the head of pancreas that may require PD.
Among these 72 patients, three were converted to conventional laparotomy due to the vascular invasion by the surrounding organs and the difficulties in tissue isolation. In two of these three patients, open surgery was performed instead the tumor invasion onto the superior mesenteric artery, adrenal glands, and post-gastric wall. The diseases were then pathologically confirmed to be the AJCC stage III and IV pancreatic cancer, respectively. According to literature and our experiences, the robotic surgical system should be applied for patients with AJCC stage I or II pancreatic cancer without portal system invasion.
In our series, the incidence of post-operative complications was 25.0% (18/72), among which the incidence of pancreatic fistula was 15.3% (11/72), the re-operation rate was 2.8% (2/72), and the mortalitywas 1.4% (1/72). These data were similar to those reported by Giullianotti et al. (16) in 134 patients who had undergone robotic pancreatic surgeries; however, since the sample size was small in our series, and the complications after robot-assisted surgeries for pancreatic cancer await further studies and larger sample sizes.
In our current study, the average post-operative hospital stay was 22.6±10.7 days, which was relatively longer than those reported in literature. That may be explained by the differences in health care systems and the lack of home-based nursing in China; as a result, the post-operative recovery was mainly done in hospitals, leading to longer hospital stay.
As shown in our clinical experiences, robotic radical resection for PDAC is feasible and safe in well selected patients. Thorough assessment of pre-operative imaging data and surgeons with rich experience in robotic surgeries are key to the success of these procedures. The robotic surgical system has advantages including small trauma and quick postoperative recovery; meanwhile, it breaks through the technical bottlenecks including complicated anastomosis and extensive lymph node dissection that are extremely difficult under the conventional laparoscopic pancreatic surgeries. Thus, the robotic surgery system has expanded the indications in the treatment of pancreatic tumors, with particularly better effectiveness for those still in AJCC stages I and II. However, its further advantages and long-term effectiveness still require validations by comparing with the open surgeries before it can be widely accepted by more clinicians and patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sudan R, Puri V, Sudan D. Robotically assisted biliary pancreatic diversion with a duodenal switch: a new technique. Surg Endosc 2007;21:729-33. [PubMed]
- Kang CM, Kim DH, Lee WJ, et al. Conventional laparoscopic and robot-assisted spleen-preserving pancreatectomy: does da Vinci have clinical advantages? Surg Endosc 2011;25:2004-9. [PubMed]
- Ntourakis D, Marzano E, De Blasi V, et al. Robotic left pancreatectomy for pancreatic solid pseudopapillary tumor. Ann Surg Oncol 2011;18:642-3. [PubMed]
- Narula VK, Mikami DJ, Melvin WS. Robotic and laparoscopic pancreaticoduodenectomy: a hybrid approach. Pancreas 2010;39:160-4. [PubMed]
- Buchs NC, Addeo P, Bianco FM, et al. Outcomes of robot-assisted pancreaticoduodenectomy in patients older than 70 years: a comparative study. World J Surg 2010;34:2109-14. [PubMed]
- Kooby DA, Gillespie T, Bentrem D, et al. Left-sided pancreatectomy: a multicenter comparison of laparoscopic and open approaches. Ann Surg 2008;248:438-46. [PubMed]
- Jayaraman S, Gonen M, Brennan MF, et al. Laparoscopic distal pancreatectomy: evolution of a technique at a single institution. J Am Coll Surg 2010;211:503-9. [PubMed]
- Vijan SS, Ahmed KA, Harmsen WS, et al. Laparoscopic vs open distal pancreatectomy: a single-institution comparative study. Arch Surg 2010;145:616-21. [PubMed]
- Hanly EJ, Talamini MA. Robotic abdominal surgery. Am J Surg 2004;188:19S-26S. [PubMed]
- Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg 2003;138:777-84. [PubMed]
- Giulianotti PC, Sbrana F, Bianco FM, et al. Robot-assisted laparoscopic middle pancreatectomy. J Laparoendosc Adv Surg Tech A 2010;20:135-9. [PubMed]
- Horiguchi A, Uyama I, Miyakawa S. Robot-assisted laparoscopic pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci 2011;18:287-91. [PubMed]
- Kim DH, Kang CM, Lee WJ, et al. The first experience of robot assisted spleen-preserving laparoscopic distal pancreatectomy in Korea. Yonsei Med J 2011;52:539-42. [PubMed]
- Lanfranco AR, Castellanos AE, Desai JP, et al. Robotic surgery: a current perspective. Ann Surg 2004;239:14-21. [PubMed]
- Daouadi M, Zureikat AH, Zenati MS, et al. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Ann Surg 2013;257:128-32. [PubMed]
- Giulianotti PC, Sbrana F, Bianco FM, et al. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc 2010;24:1646-57. [PubMed]