Progression of targeted therapy in advanced cholangiocarcinoma
Introduction
cholangiocarcinoma (CCA) is the second most common primary hepatic malignancy. It was described for the first time by Durand-Farde in 1840 (1). Till 1911 hepatic malignancy had been divided into hepatocellular carcinoma (HCC) and CCA (2). The CCA can also be further classified as intrahepatic cholangiocarcinoma (IHCC) and extrahepatic cholangiocarcinoma (EHCC). The incidence of CCA is increasing every year, especially in South America and Asia. The new diagnosed CCA patients per year are about 16,000 in Japan and 5,000 in America (3-5). We pay more attention to CCA recently for the bad prognosis and bad effect to all kinds of treatments, especially for the advanced patients. While it is still very difficult to expand randomized controlled clinical trials because of its rare incidence and small quantity of patients in one center, there is no good or standard first line chemotherapy for CCA for most of advanced patients. In Japan they made the gemcitabine alone or S1 as the first line regimen for advanced CCA according to one phase II clinical trial outcome (6). While now according to the recent randomize-controlled researches, it was proved that combination with gemcitabine and platinum is superior to gemcitabine alone and the combination can bring some benefits on overall survival (7,8). There are more and more studies which support the chemotherapy combination and targeted therapy with chemotherapy. At the same time in some pathology researches found there are many bio-markers expression or mutation in CCA patients such as epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), and human epidermal growth factor receptor 2 (HER2), MEK (Mitogen-activated protein kinase) et al. (9-14). These are the basis to do further studies about targeted therapy in CCA. There are many clinical trials which had proved the efficiency and rationality of targeted therapy in CCA.
The rationality of targeted therapy in CCA
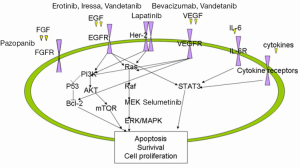
With the success of experiences in lung, breast, melanoma, GIST (Gastrointestinal stromal tumor) and colorectal cancer treated by targeted therapies and new target agents, more and more researches aim to look for the special bio-markers and ideal therapies in CCA. According to the bio-markers study we can find out the rationality of targeted therapy in CCA (Figure 1).
Firstly, it is the expression of EGFR, VEGF and HER 2 in CCA. In 1988 Nonomura found the EGF and EGFR expressed in CCA (10). Then a retrospective study about these molecules had been done by Yoshikawa (11). The study included 236 CCA patients. They found EGFR, VEGF, and HER2 over expression. The percentage of EGFR over expression was 27.4% in IHCC and 19.2% in EHCC, VEGF was 53.8% in IHCC and 59.2% in EHCC, HER2 was 0.9% in IHCC and 8.5% in EHCC. Multivariate analysis showed that EGFR expression was a significant prognostic factor and also a risk factor for tumor recurrence in IHCC. From this study it seems that EGFR expression is associated with tumor progression and VEGF expression may be involved in haematogenous metastasis in CCA. Nakazawa did another study for detecting the promising bio-markers in CCA (12). The study included 221 biliary tract carcinomas. All of tissues were analyzed by IHC (immunohistochemistry) firstly and positively stained tumors were further examined for gene amplification by fluorescence in situ hybridization. They found ErbB-2, EGFR and Met over expressions in biliary duct tumor. Over expressions of ErbB-2 and EGFR were associated with gene amplifications with high frequency (77%) except MET. Tannapfel also found BRAF gene and KRAS gene mutations in CCA (15). During 69 CCA patients there were 22% of BRAF gene mutation and 45% of KRAS mutation. Both of these mutations had no relation to tumor progression. While at the same time MAPK protein was over expressed with BRAF mutation. There are still a lot of other studies about the IL-6, STAT3 and MEK expression in CCA (16,17). Recently, Mitesh et al. (18) had published one paper. They tried to screen for potent target agents in six advanced CCA and found recurrent translocation of FGFR2 in three patients. Treated these patients with FGFR inhibitor, there had tumor shrinkages in these three patients. At same time they also found a non-FGFR fusion patient who had ERRFI1 gene function loss which is a direct negative regulator of EGFR activation. This patient had a rapid and robust tumor regression when treated with Tarceva. These showed that maybe the EGFR and FGFR pathway would be the potent targeted in CCA. Because they just checked six patients in every detail such as whole genome sequencing, exome sequencing and RNA sequencing also. It seemed promising and these targets need more studies to confirm. All of these studies showed maybe there are some potential bio-markers which can indicate the rationality of new targeted therapy and at the same time we can make a good clinical trial to look for promising treatments.
Targeted therapy in clinical trials
Single targeted therapy
Target Agents which had been studied on cholangiocarcinoma are Erotinib, Lapatinib, Sorafenib and selumetinib. Table 1 shows those promising treatments in CCA (Table 1).
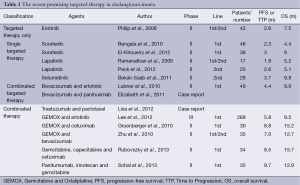
Full table
Erlotinib
In 2005 Philip had studied a phase II clinical trial on CCA (19). They enrolled 42 advanced CCA patients. The median age was 67 years. 57% percent of patients had been received chemotherapies before. All patients’ performance status (PS) according to Eastern Cooperative Oncology Group (ECOG) was 1. Patients were treated with erlotinib 150 mg/d, 28 days as one circle. All of patients had good tolerance during the first circle. Only 3 (7%) patients had toxicity-related dose reductions of erlotinib. The median duration for the treatment was 4.4. There was 3 patients had partial response (PR) by Response Evaluation Criteria in Solid Tumors Group Classification (RECIST). A total of 17 (43%) patients were SD. The median Time to Progress (TTP) was 2.6 months and median overall survival (OS) was 7.5 months. A total of 36 assessable patients had been tested for HER1/EGFR expression by immunohistochemistry. A total of 29 (81%) patients were positive. Among those positive cases there were 7 patients who had disease progression after 24 weeks and at the same time all of negative cases had disease progression in 24 weeks. In this study they also had done the expression and classified it as 0,1,2,3. They founded that it did not affect the analysis. That means it does not matter how strongly the Gene expressed and it just has relation to whether EGFR had be expressed.
Sorafenib
There are two important studies. One was done by Bengala (20). This phase II clinical trial enrolled 46 advanced CCA patients who had been treated with chemotherapies before. After Sorafenib treatment, there was only 1 (2.2%) patient with PR, 12 (30.4%) patients with SD. The median TTP was 2.3 months and the median OS was 4.4 months. The other was SWOG0514 (21). Sorafenib was used as the first-line treatment for CCA patients. 36 patients were enrolled and no one got PR. 10 (32%) patients got SD. While the median TTP was 3 months and the median OS was 9 months. This study proved Sorafenib alone can achieve the good result as combination treatment of gemcitabine and capecitabine in SWOG0202 which showed 27% SD and 7 months OS. These studies also proved that Sorafenib could be a promising target agent in CCA.
Lapatinib
In preclinical trials it had been proved over expressions of EGFR and HER2 and with inhibitors in these target bio-markers had showed some benefits. In followed clinical trials it is disappointed. Peck tried to enroll 25 patients for the lapatinib treatment while the study had to been stopped for there was no one PR when the OS arrived 5.1 months (22). To mention that there was no patient who had EGFR and HER2 mutation. Rammanatha did another study while there was still no PR with 5.2 months OS (23). Maybe we can think this result is due to the enrolled patients without EGFR or HER 2 mutation and over expression. If we can do other study with the special bio-markers positive patients we could get the positive result.
Selumetinib
This is a promising inhibitor to mitogen-activated protein kinase (MEK-1/2). It showed very good outcomes. In 2011 Journal of Clinical Oncology had published a phase II clinical trial (24). During the study there were 28 metastatic biliary cancer patients enrolled. The median age was 55.6 years. A total of 3 patients (12%) had a confirmed objective response. A total of 17 patients (68%) had stable disease (SD), in those stable diseases there were 14 (56%) patients whose SD were over 16 weeks. Median PFS was 3.7 months and OS was 9.8 months. This is one of the best outcomes for the advanced CCA patient with the single targeted therapy. They also found that response to selumetimib was associated with extracellular signal-related kinase (pERK) staining. Pardo also repeated the same study with 20 patients (25). The median OS was 9.8 months. These studies firstly proved that MEK inhibitor had affected signaling pathways in the carcinogenesis of CCA patients.
They also found patients had acceptable tolerability to selumetinib and no special adverse drug reactions were recorded compared with other targeted therapy before.
Targeted therapy combination
Combination with bevacizumab and TKI
Lubner SJ did such kind of study (26). During the 49 evaluable patients, there were six (12%) patients who had a confirmed PR and 25 (51%) patients valued as stable disease. Median OS was 9.9 months, and TTP was 4.4 months. During the study there were 2 patients died. One died of ischemic cerebral disease and the other one died of thrombotic disease. While the OS is longer than those treated with Erotinib along. At the same time it had the same finding as in lung cancer researches that patients who had KRAS mutations would have bad reactions to Erotinib.
Combination with bevacizumab and panituximab
This is just a case report (27). While this combination treatment was firstly used in bile duct carcinoma and it had very dramatic response. The patient was 76-year-old male and diagnosed as advanced bile duct carcinoma. The PS was 3 when he was admitted into the hospital. After treated with bevacizumab and panituximab every two weeks, his PS was 0 and SUV of targeted organs also decreased obviously. The specialty of this case was that those patients even in bad status could get benefit from such kind of targeted therapy. The tolerance was still good. We can also find that the good response maybe has some relation to his EGFR mutation.
Combination with targeted therapy and chemotherapy
Combination with trastuzumab and paclitaxel
This is the first reported case that advanced CCA patient got the dramatic response with the combination treatment of trastuzumab and paclitaxel (28). The patient was a 45-year-old. By the biopsy she was diagnosed with metastatic CCA. After several failures of conventional chemotherapies she was suggested to test the HER2. By FISH analysis HER2 amplification was found so that they decided to give this patient the combination treatment with weekly trastuzumab and paclitaxel. After 9 weeks of therapy CT scan showed the dramatic regression of both lung metastases. This study also showed that optimal personalized treatment with the knowledge of bio-markers situations could be considerate.
Combination with target agents and Gemcitabine and Oxlaliplatine (GEMOX)
It includes Cetuximab, bevacizumab, erlotinib, lapatinib, selumetinib and so on. While till now the promising one is combination with cetuximab.
In 2010 Gruenberge published a phase II study (29). 30 advanced CCA patients without surgery were enrolled. All patients received intravenous infusions of 500 mg/m2 cetuximab on day 1, after 30 minutes without adverse reaction then continued with gemcitabine and oxaliplatin, every 2 weeks for 12 cycles. A total of 19 patients (63%) got objective response and it was amazing that three (10%) patients achieved complete response. Nine patients underwent potentially curative secondary resection after therapy. A total of 8 patients had tumour shrinkage by at least 40% of the sum of the longest diameter of target lesions which was evaluated by PET. Overall response rate achieved to 80%. PFS for all patients was 8.8 months and for those 9 patients received surgery was 15.2 months. In this study only three patients had KRAS mutations. In these 3 patients there were two IHCC and another one was EHCC. Those two patients had PR and the other one got SD. One of these 3 patients got the surgery after four circles treatment. There was also a positive correlation between the grade of skin rash and the response. Rubovszky did another study as the same with cetuximab, gemcitabine and capecitabine (30). It also had 2 CR, 86.5% DCR and 15.7 months OS.
Another phase 3 clinical trial evaluated the efficiency of combination with erlotinib and GEMOX (31). A total of 268 patients were enrolled. All of patients were in advanced stage and chemo-naive or without chemotherapy in 6 months. Comparing to chemotherapy alone group, the chemotherapy plus erlotinib group had significantly objective response (40 patients vs. 21 patients; P=0.005), but median overall survival was the same with 9.5 months. Zhu AX did another phase 2 clinical trial as the same (32). A total of 35 patients were enrolled and evaluable for efficacy and toxicity. It seemed that patients had good tolerance and the median PFS was 7.0 months. The important founding in this trial was that they found the change in SUV(max) was a significant predictor of PFS.
Combination with panitumumab, irinotecan and gemcitabine
Sohal DP et al. did one phase II study (33). There were 35 patients with advanced CCA who treated with panitumumab, gemcitabine and irinotecan on day 1 and 8, every 21 days as one cycle. Mutational analyses of EGFR, KRAS and BRAF were carried out when feasible. As a result, two patients had CR, 9 had PR, and 15 had SD for a disease-control rate of 74% (by RECIST) in 28 assessable patients. Two patients went on to have surgical resection. The median PFS was 9.7 months and the median OS was 12.9 months. No EGFR or BRAF mutations were identified; there were 7 KRAS mutations while there was no difference in OS by KRAS status.
Necessary for detecting prior to clinical trials
Since CCA is seldom, it is very difficult to collect so many data. Before we start some new clinical trial we’d better detect some bio-markers if we can. Nearly all tumor groups have made the same error being made by CCA physicians now, they attempt to treat all cancers the same. In breast cancer, physicians clinically test for HER2 prior to therapy selection; in lung cancers, physicians test for EGFR, ALK, ROS1; for GIST tumors, clinicians test for KIT and PDGFRA mutations; and in melanoma, patients undergo BRAF testing prior to therapy selection. This data clearly shows that CCA tumors need to be profiled before selecting a targeted therapy to assure that the patient gets the best possible treatment. Failure to do so will result in a low percentage of patients responding to any targeted therapy, because they don’t know the target! Maybe we should remember, as to targeted therapy, there will be No Target, No Benefit.
Conclusions
CCA is a kind of malignancies with bad overall survival. There is no good standard treatment till now. With its fewer patients in one study center cooperation between many centers is necessary to get more accurate and optimal result. With the knowledge of special bio-markers in CCA and new target agents more and more, it seems that combination with targeted therapy and chemotherapy is promising. Detection of special bio-markers in CCA is very important. It seems that the study of tumor pathway especially of EGFR and MEK pathway could be more indicative to optimal targeted therapy. In the future with multi-center randomized controlled clinical trials which designed according to the special bio-markers we could get more confident evidences and get best guidelines in cholangiocarcinoma treatment.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Renshaw K. Malignant neoplasms of the extrahepatic biliary ducts. Ann Surg 1922;76:205-21. [PubMed]
- Goldzieher M, von Bokay Z. Der primaere leberkrebs. Virchows Arch 1911;203:75-131.
- Randi G, Malvezzi M, Levi F, et al. Epidemiology of biliary tract cancers: an update. Ann Oncol 2009;20:146-59. [PubMed]
- Lazaridis KN, Gores GJ. Cholangiocarciinoma. Gastroenterology 2005;128:1655-67. [PubMed]
- Aljiffry M, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of cholangiocarcinoma: 1990-2009. World J Gastroenterol 2009;15:4240-62. [PubMed]
- Furuse J, Takada T, Miyazaki M, et al. Guidelines for chemotherapy of biliary tract and ampullary carcinomas. J Hepatobiliary Pancreat Surg 2008;15:55-62. [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [PubMed]
- Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 2010;103:469-74. [PubMed]
- Thomas MB. Biological characteristics of cancers in the gallbladder and biliary tract and targeted therapy. Crit Rev Oncol Hematol 2007;61:44-51. [PubMed]
- Nonomura A, Ohta G, Nakanuma Y, et al. Simultaneous detection of epidermal growth factor receptor (EGF-R), epidermal growth factor (EGF) and ras p21 in cholangiocarcinoma by an immunocytochemical method. Liver 1988;8:157-66. [PubMed]
- Yoshikawa D, Ojima H, Iwasaki M, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer 2008;98:418-25. [PubMed]
- Nakazawa K, Dobashi Y, Suzuki S, et al. Amplification and overexpression of c-erbB-2, epidermal growth factor receptor, and c-met in biliary tract cancers. J Pathol 2005;206:356-65. [PubMed]
- Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408-17. [PubMed]
- Yoshikawa D, Ojima H, Kokubu A, et al. Vandetanib (ZD6474), an inhibitor of VEGFR and EGFR signalling, as a novel molecular-targeted therapy against cholangiocarcinoma. Br J Cancer 2009;100:1257-66. [PubMed]
- Tannapfel A, Sommerer F, Benicke M, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut 2003;52:706-12. [PubMed]
- Andersen JB, Spee B, Blechacz BR, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology 2012;142:1021-1031.e15.
- Isomoto H, Kobayashi S, Werneburg NW, et al. Interleukin 6 upregulates myeloid cell leukemia-1 expression through a STAT3 pathway in cholangiocarcinoma cells. Hepatology 2005;42:1329-38. [PubMed]
- Borad MJ, Champion MD, Egan JB, et al. Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. PLoS Genet 2014;10:e1004135. [PubMed]
- Philip PA, Mahoney MR, Allmer C, et al. Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol. 2006;24:3069-74. [PubMed]
- Bengala C, Bertolini F, Malavasi N, et al. Sorafenib in patients with advanced biliary tract carcinoma: a phase II trial. Br J Cancer 2010;102:68-72. [PubMed]
- El-Khoueiry AB, Rankin CJ, Ben-Josef E, et al. SWOG 0514: a phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. Invest New Drugs 2012;30:1646-51. [PubMed]
- Peck J, Wei L, Zalupski M, et al. HER2/neu may not be an interesting target in biliary cancers: results of an early phase II study with lapatinib. Oncology 2012;82:175-9. [PubMed]
- Ramanathan RK, Belani CP, Singh DA, et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother Pharmacol 2009;64:777-83. [PubMed]
- Bekaii-Saab T, Phelps MA, Li X, et al. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol 2011;29:2357-63. [PubMed]
- Prado CM, Bekaii-Saab T, Doyle LA, et al. Skeletal muscle anabolism is a side effect of therapy with the MEK inhibitor: selumetinib in patients with cholangiocarcinoma. Br J Cancer 2012;106:1583-6. [PubMed]
- Lubner SJ, Mahoney MR, Kolesar JL, et al. Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: a phase II Consortium study. J Clin Oncol 2010;28:3491-7. [PubMed]
- Riley E, Carloss H. Dramatic response to panitumumab and bevacizumab in metastatic gallbladder carcinoma. Oncologist. 2011;16:e1-2. [PubMed]
- Law LY. Dramatic response to trastuzumab and paclitaxel in a patient with human epidermal growth factor receptor 2-positive metastatic cholangiocarcinoma. J Clin Oncol 2012;30:e271-3. [PubMed]
- Gruenberger B, Schueller J, Heubrandtner U, et al. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: a phase 2 study. Lancet Oncol 2010;11:1142-8. [PubMed]
- Rubovszky G, Láng I, Ganofszky E, et al. Cetuximab, gemcitabine and capecitabine in patients with inoperable biliary tract cancer: a phase 2 study. Eur J Cancer 2013;49:3806-12. [PubMed]
- Lee J, Park SH, Chang HM, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2012;13:181-8. [PubMed]
- Zhu AX, Meyerhardt JA, Blaszkowsky LS, et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol 2010;11:48-54. [PubMed]
- Sohal DP, Mykulowycz K, Uehara T, et al. A phase II trial of gemcitabine, irinotecan and panitumumab in advanced cholangiocarcinoma. Ann Oncol 2013;24:3061-5. [PubMed]