Pancreatic cancer incidence and mortality patterns in China, 2011
Introduction
Pancreatic cancer is one of the most fatal malignancies with an overall survival rate of only about 5%. Surgery is the most effective therapy to cure this disease, but less than 20% of patients present with early disease onset (1). With the estimated 337,872 new cases and 330,391 deaths, pancreatic cancer is the 12th common cancer and the 7th leading cause of cancer deaths worldwide (2). According to the estimation by the National Central Cancer Registry of China (NCCR), the pancreatic cancer incidence rate was 7.28/100,000 (males 8.24/100,000, females 6.29/100,000), ranking 7th among all cancers in China in 2009. The mortality rate of pancreatic cancer was 6.61/100,000 (males 7.45/100,000, females 5.75/100,000), ranking 6th among all cancer deaths at the same time (3). In the mid of 2014, total 234 registries covering 221 million, accounting for 16.4% of national populations reported registration data of the year 2011 to NCCR. This paper analyzed the pancreatic cancer incident and death status in China in 2011.
Materials and methods
Data source
The NCCR is responsible for cancer data collection, evaluation and publication from local population-based cancer registries. The cancer information was reported to the cancer registries from local hospitals and community health centers, including the Basic Medical Insurances for urban residents and the New-Rural Cooperative Medical System. The Vital Statistical Database was linked with the cancer incidence database for identifying cases with death certificate only (DCO) and follow-up. By June 1, 2014, 234 cancer registries (98 cities and 136 counties) from 31 provinces submitted 2011 data to the NCCR. Data covered about 221,390,275, accounting for 16.43% of whole national population in 2011. Among them, there were 177 population-based cancer registries whose data quality met the quality criteria required by NCCR, distributed in 28 provinces (77 in urban and 100 in rural areas), and covered 175,310,169 population accounting for about 13.01% of the whole Chinese population, including 88,655,668 males and 86, 654,501 females, 98,341,507 in urban and 76,968,662 in rural areas. All cancer cases were classified according to the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) and the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10). Invasive cases of pancreatic cancer (ICD10: C25) were extracted and analyzed from the overall cancer database.
Population data
The population was estimated based on the fifth National Population Census data [2000] provided by the National Statistics Bureau of China, taking into account of the changes of age composition, gender ratio and the proportion of urban and rural transformation released by the National Bureau of Statistics (http://data.stats.gov.cn/). The national populations in 2011 were stratified by area (urban/rural), gender (male/female) and age groups (0-, 1-4, 5-84 by 5 years, 85+ years). The changes of age-specific death probability were also adjusted when calculating population. Linear changes were assumed in each age group between the fifth and sixth population census.
Quality control
According to “Guideline of Chinese Cancer Registration”, we checked the data quality using the inclusion criteria in “Cancer Incidence in Five Continents Volume IX” (4), which was required by the International Association for Cancer Registry (IACR) and the International Agency for Research on Cancer (IARC) (5). We used software including MS-Excel, Statistical Analysis System (SAS) and IARC Tools issued by the IARC/IACR for data check and evaluation. The data were included in the present analysis if they met the following criteria: morphological verification (MV%) higher than 66%, percentage of cancer cases identified with death certification only (DCO%) less than 15%, and mortality to incidence ratio (M/I) between 0.6 and 0.8.
Statistical analysis
Incidence and mortality rates were calculated by area, gender and age groups. The number of new cases and deaths were estimated using the 5-year age-specific cancer incidence/mortality rates and the corresponding populations. The Chinese population in 2000 and World Segi’s population were used for age-standardized rates. The cumulative risk of developing or dying from cancer before 75 years of age (in the absence of competing causes of death) was calculated and presented as a percentage. Software including MS-Excel and IARCcrgTools2.05 issued by IARC and IACR was used for data checking and evaluation. SAS software (SAS Institute Inc., Cary, USA) was used to calculate the incidence and mortality rates.
Results
Quality evaluation
The coverage population of all 177 cancer registries was 175,310,169. Pancreatic cancer M/I ratio in all cancer registry areas was 0.91 (males 0.90 and females 0.93). MV% of pancreatic cancer was 40.52% (males 41.17% and females 39.69%). DCO% was 4.33% (males 4.44% and females 4.19%). Pancreatic cancer M/I ratio in urban areas was 0.93, which was higher than that in rural areas (0.88). Likewise, MV% (41.94%) and DCO% (4.39%) in urban areas was higher than MV% (37.59%) and DCO% (4.22%) in rural areas (Table 1).
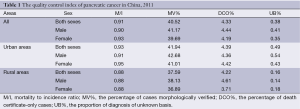
Full table
Incidence rate
In 2011, the crude incidence rate of pancreatic cancer in the registry areas was 5.96/100,000 (6.57/100,000 for males and 5.32/100,000 for females), accounting for 2.38% of all cancers. The age-standardized rates were 4.27/100,000 and 4.23/100,000, respectively, after being standardized by the age structures of Chinese and the world populations. For patients aged 0-74 years, the cumulative incidence rate was 0.50% and aged 35-64 years, and the truncated age-standardized rate (TASR) was 5.68/100,000. In urban areas, the incidence rate was 7.03/100,000 (7.75/100,000 for males and 6.28/100,000 for females), while in rural areas, it was 4.84/100,000 (5.34/100,000 for males and 4.31/100,000 for females). Standardized by the age structures of China and the world, in urban areas, the age-standardized rates were 4.94/100,000 and 4.90/100,000. Both crude incidence rates and age-standardized incidence rates in urban areas were higher than those in rural areas (Table 2).
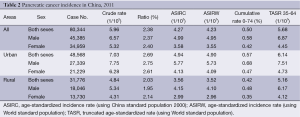
Full table
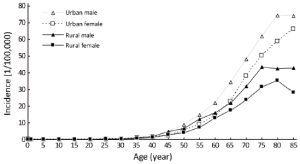
Age-specific incidence rate
The age-specific incidence rate of pancreatic cancer was low before 40 years old, and dramatically increased after then. They reached a peak at the age group of 80- years which were 74.50/100,000 for urban males, while at the age group of 75- years which were 43.44/100,000 for rural males. For urban females, the incidence rate of pancreatic cancer reached a peak at the age group of 85+ years which were 66.18/100,000 in urban areas, whereas at the age group of 80- years in rural areas which were 35.26/100,000 in urban areas.
The age-specific incidence rate of pancreatic cancer among males was higher in urban areas than that in rural areas in all age groups, except for the age group of 35-49 years. For females, it had the same trend except for the age groups of 25-39 years (Table 3 and Figure 1).
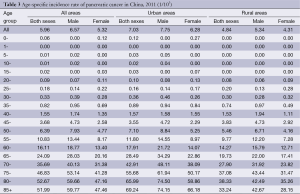
Full table
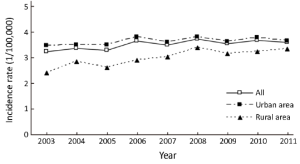
Incidence rates of pancreatic cancer from 2003 to 2011
There were 33 registries kept submitting data to NCCR from 2003 to 2011. About incidences of pancreatic cancer, there were fluctuations in the different regions and genders. The incidence rate of pancreatic cancer increased from 3.24/100,000 in 2003 to 3.59/100,000 in 2011 with an annual percentage change (APC) of 1.44 while the APC of males was 1.48. There was no statistically significant in APC of females. In urban areas, the rate was 1.05 times higher in 2011 than that in 2003, but the APC showed no statistically significant in both sexes. Meanwhile, the incidence rate increased 1.39 times from 2003 to 2011 in rural areas with an APC of 3.78 (Table 4 and Figure 2).
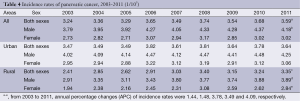
Full table
Mortality
The crude mortality rate of pancreatic cancer was 5.40/100,000 (5.88/100,000 in males and 4.89/100,000 in females). The China standardized rate was 3.81/100,000, compared with the world standardized rate of 3.79/100,000. The cumulative rate (0-74 years old) was 0.44% and aged 35-64 years, the TASR was 4.74/100,000. The crude mortality rate of pancreatic cancer in urban areas was 6.47/100,000 (7.01/100,000 in males and 5.91/100,000 in females). The age-standardized mortality rates based on the Chinese standard population (ASMRC) and the world standard population (ASMRW) were 4.48/100,000 and 4.47/100,000, respectively. Among patients aged 0-74 years in urban, the cumulative mortality rate was 0.51%, and aged 35-64 years, the TASR was 5.25/100,000. In rural areas, the crude mortality rate was 4.27/100,000 (4.70/100,000 in males and 3.82/100,000 in females). The ASMRC was 3.08/100,000 and the ASMRW was 3.07/100,000. The cumulative mortality (0-74 years) was 0.36%, and aged 35-64 years, the TASR was 4.17/100,000. Urban areas had a higher mortality than rural areas (Table 5).
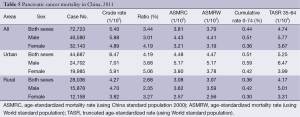
Full table
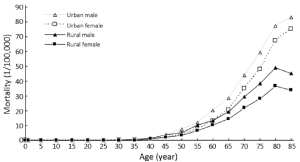
Age-specific mortality rate
The trend in the age-specific mortality rate of pancreatic cancer in urban areas was similar to that in rural areas. The age-specific mortality rate of pancreatic cancer was relatively low in the population younger than 40 years old. There was a dramatic increase in the mortality rate after 50 years old. In urban areas, the age-specific mortality rate of pancreatic cancer reached a peak in the age group of 85+ years whereas it reached a peak at the age group of 80- years in rural areas.
The age-specific mortality rate of pancreatic cancer among males was higher in urban areas than in rural areas in all age groups, except for the age group of 35-49 years. For females, it had the same trend except for the age groups of 20-29 years (Table 6 and Figure 3).
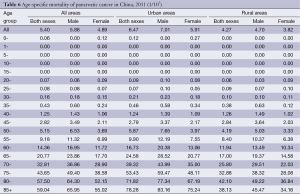
Full table
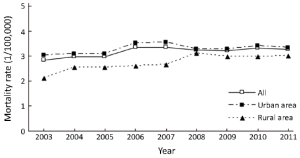
Mortality rates of pancreatic cancer from 2003 to 2011
The data of 33 cancer registries showed that between 2003 and 2011, the mortality rates of pancreatic cancer had an approximately 1.14-fold increase, from 2.85/100,000 in 2003 to 3.26/100,000 in 2011, with an APC of 1.68. During the period 2003-2011, the mortality rates increased from 3.31/100,000 to 3.71/100,000 in males with an APC of 1.63, and from 2.41/100,000 to 2.83/100,000 in females with an APC of 1.68. The APC showed no statistically significant in urban areas. In rural areas, the APC was 4.09 in both sexes. Moreover, the APC of rural male was 4.32, while the rural females’ APC was 3.73 (Table 7 and Figure 4).
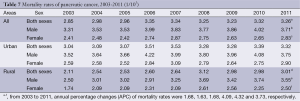
Full table
Discussion
With poor prognosis, pancreatic cancer is one of malignant tumors with the highest mortality rates worldwide. In the world, approximately 330,391 subjects died from pancreatic cancer per year, making it the 7th leading cause of cancer-related death (2), which is seriously harmful for the health of human beings. Pancreatic cancer has an overall 5-year survival of less than 5% because there are no reliable tests for early diagnosis and no effective therapies for the metastatic form of pancreatic cancer. The only curative treatment for pancreatic cancer is surgical resection which alone can improve 5-year survival to 10%. However, 80% of pancreatic adenocarcinoma is not resectable in the patients with clinical symptoms (6).
This study about pancreatic cancer reported that the incidence of pancreatic cancer was 5.96/100,000, and the mortality rate was 5.40/100,000 in China in 2011. Compared with the data of GLOBOCAN 2012, the male incidence of pancreatic cancer in China (ASIRW 4.50/100,000) was higher than the average level of developing countries (3.30/100,000), but lower than the world (4.90/100,000) and developed countries (8.60/100,000). From 2003 to 2011, the APC incidence and mortality rates of both sexes were 1.44 and 1.68 in China. The APC of male was 1.48. However, there was no statistically significant in APC of females. In urban areas, the APC incidence and mortality rates showed no statistically significant yet. In rural areas, the APC incidence and mortality rates were 3.78 and 4.09 in both sexes.
Pancreatic cancer is related to genetic susceptibility and dietary factors, and closely associated with lifestyles and body status. Genetic risk factors were believed to play a major role. Approximately 10% of pancreatic cancer was estimated to have familial inheritance (7). Analyses of family history of pancreatic cancer including 1,183 cases and 1,205 controls showed that a family history of pancreatic cancer in a parent, sibling or child was associated with increased risk of pancreatic cancer [multivariate-adjusted odds ratios (ORs) =1.76; 95% confidence interval (95% CI): 1.19-2.61] (8).
Several studies investigated the relation between diabetes and pancreatic cancer. The proportion of pancreatic cancer patients who also have hyperglycemia or diabetes has previously been under appreciated; new data showed that up to 80% are either hyperglycemic or diabetic and this can be evident in the pre-symptomatic phase (9). Some early studies showed that new-onset diabetes had the strongest association with pancreatic cancer and was largely responsible for the link between diabetes and pancreatic adenocarcinoma (10). Another study found that diabetes was an important risk factor for pancreatic cancer, of which OR was 2.69 (95% CI: 1.51-4.77), which is supported by a veterans system study, where diabetic patients developed pancreatic cancer more easily, compared with non-diabetic patients, resulting in a hazard ratio of 2.17 (95% CI: 1.70-2.77) (11).
Some other factors of body status, including obesity and pressure, were observed to be associated with the pancreatic cancer risk. Obesity has been proposed as additional risk factors for pancreatic cancer. Several studies suggested that obesity could increase the pancreatic cancer risk, which was found to be around 20% higher for obese compared to normal weight individuals (12), although the possibility of confounding cannot be excluded. As to the dietary factors, a study supported fruit consumption to reduce pancreatic cancer risk (OR =1.73 for consumption of 1-2 vs. more than 3 times/week; 95% CI: 1.05-2.86) and indicated that high consumption of meat was related to an elevated risk (OR =0.59 for consumption of 1-2 vs. more than 3 times/week; 95% CI: 0.35-0.97). Tea intake (OR =0.49; 95% CI: 0.30-0.80) was associated with a half reduction in risk of pancreatic cancer. Reduced vegetable consumption (P trend: 0.04) was significant related to pancreatic cancer (13).
Cigarette smoking is the best established risk factor for pancreatic cancer (14,15). In the International Pancreatic Cancer Cohort Consortium nested case-control study (16), which included 1,481 cases and 1,539 controls, the relative risk (RR) was 1.1 (95% CI: 0.9-1.3) for former smokers and 1.8 (95% CI: 1.4-2.3) for current smokers. Significant trends in risk were observed with increased number of cigarettes smoked and duration of exposure, the RR being 1.75 for 30 or more cigarettes smoked per day and 2.1 for 50 or more years of smoking, whereas the RR for those who had quit smoking for >15 years was similar to that of never smokers. A meta-analysis of 82 cohort and case-control studies published between 1950 and 2007 (17) reported a summary RR of pancreatic cancer of 1.7 (95% CI: 1.6-1.9) for current smokers and of 1.2 (95% CI: 1.1-1.3) for former smokers.
When commenting on our results, some limitations must be kept in mind. Data only covered about 16.43% of whole national population in 2011. But it is a true reflection of the malignant situation.
Conclusions
Pancreatic cancer burden is getting serious with the low survival rates. Risk factors, such as smoking, diabetes, obesity and bad dietary habit, maintain high level in Chinese. Pancreatic cancer control strategies, including health education, health promotion, early detection and cancer screening, should be treated as priority in public health.
Acknowledgements
We gratefully acknowledged the cooperation of all the population-based cancer registries in providing cancer statistics, data collection, sorting, verification and database creation. The authors assume full responsibility for analyses and interpretation of these data.
Disclosure: The authors declare no conflict of interest.
References
- Sutton JM, Abbott DE. Neoadjuvant therapy for pancreas cancer: past lessons and future therapies. World J Gastroenterol 2014;20:15564-79. [PubMed]
- GLOBOCAN 2012. Lyon: IARC. Available online: http://globocan.iarc.fr/
- Chen WQ, Liang D, Zhang SW, et al. Pancreatic cancer incidence and mortality patterns in china, 2009. Asian Pac J Cancer Prev 2013;14:7321-4. [PubMed]
- Curado MP, Edwards B, Shin HR, et al. Cancer Incidence in Five Continents. Vol. IX. Lyon: IARC Scientific Publications, 2008.
- Ferlay J, Burkhard C, Whelan S, et al. Check and conversion programs for cancer registries. Lyon: IARC Technical Report 2005.
- Jones OP, Melling JD, Ghaneh P. Adjuvant therapy in pancreatic cancer. World J Gastroenterol 2014;20:14733-46. [PubMed]
- Ghiorzo P. Genetic predisposition to pancreatic cancer. World J Gastroenterol 2014;20:10778-89. [PubMed]
- Jacobs EJ, Chanock SJ, Fuchs CS, et al. Family history of cancer and risk of pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Int J Cancer 2010;127:1421-8. [PubMed]
- Zhang C, Yang G, Ling Y, et al. The early diagnosis of pancreatic cancer and diabetes: what's the relationship? J Gastrointest Oncol 2014;5:481-8. [PubMed]
- Hemminki K, Li X. Familial and second primary pancreatic cancers: a nationwide epidemiologic study from Sweden. Int J Cancer 2003;103:525-30. [PubMed]
- Silverman DT. Risk factors for pancreatic cancer: a case-control study based on direct interviews. Teratog Carcinog Mutagen 2001;21:7-25. [PubMed]
- Yacoub A, Siegel E, Makhoul I. Pancreatic cancer and diabetes mellitus: A retrospective cohort study. J Clin Oncol 2011;29:4102.
- Liu SZ, Chen WQ, Wang N, et al. Dietary factors and risk of pancreatic cancer: a multi-centre case-control study in China. Asian Pac J Cancer Prev 2014;15:7947-50. [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum 2004;83:1-1438. [PubMed]
- Secretan B, Straif K, Baan R, et al. A review of human carcinogens--Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol 2009;10:1033-4. [PubMed]
- Lynch SM, Vrieling A, Lubin JH, et al. Cigarette smoking and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Am J Epidemiol 2009;170:403-13. [PubMed]
- Iodice S, Gandini S, Maisonneuve P, et al. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg 2008;393:535-45. [PubMed]