Decline of serum CA724 as a probable predictive factor for tumor response during chemotherapy of advanced gastric carcinoma
Introduction
Gastric carcinoma (GC) has emerged as a major public health problem and one of the most common malignancies in the world (1). Although its morbidity is sharply decreasing in the Western, GC keeps a high incidence in some eastern countries, such as Japan, Korea and China (2). Owing to its poor outcome, GC presents one of the great therapeutic challenges for oncologists (3). Palliative chemotherapy is the main option for GC patients with advanced stages to encourage survival outcomes and better quality of life (4,5). Therefore, it would be crucial to evaluate chemotherapy efficacy earlier and avoid ineffective treatment in time. However, imaging techniques used to estimate therapeutic effect are usually performed after various cycles of chemotherapy by macroscopic alterations of tumor diameter (6). Accordingly, to find an easier and cheaper tool to monitor the effects of chemotherapy would be extremely urgent.
Tumor biomarkers expressed in different biological tissues may reflect relative tumor burden and aggressive biology (7). A meta-analysis showed that carbohydrate antigen 724 (CA724) was the most correlative serum tumor biomarker for the detection of GC in Chinese population (8). However, by now, there are few investigations focusing on CA724 for predicting the tumor response during chemotherapy. We examined the predictive value of serum CA724 for objective responses to the chemotherapy in patients with advanced GC.
Patients and methods
Patient selection
A group of 98 patients, at the Yancheng City No. 1 People’s Hospital between October 2008 and October 2013, was included in the study meeting the following criteria: (I) pathologically confirmed and previously untreated adenocarcinoma arising from the stomach; (II) clinical tumor stage IIIc-IV, on the basis of the sixth edition of the American Joint Committee on Cancer (AJCC) (9); (III) inoperable locally advanced or metastatic disease with measurable lesions by computed tomography (CT) scan; (IV) an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0-2; (V) an estimated life expectancy of at least 3 months; (VI) patients with adequate bone marrow, liver, heart, and renal functions; (VII) written informed consents were given before treatment; (VIII) the medical ethics committee of the Yancheng City No. 1 People’s Hospital approved this study.
Treatment schedule
All patients were treated with an FLO regimen every 4 weeks of oxaliplatin 130 mg/m2 on day 1 and leucovorin 50 mg/m2 on days 1-5, each as an IV infusion followed by 5-fluorouracil 500 mg/m2 as a 24-h continuous infusion on days 1-5. Treatment was repeated at most six cycles until disease progression, unacceptable toxicity, or withdrawal by the patient.
Evaluation of objective response
The clinical tumor response was assessed every two cycles of chemotherapy, using the RECIST criteria version 1.1 (10): complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). The evaluation was performed 3 weeks after the treatment. Objective response rate (ORR) (%) = (CR + PR)/98 × 100%.
Serum assays for CA724
Venous blood samples were collected before chemotherapy and on the evaluation day after informed consent. Serum CA724 was measured by an electrochemiluminescence immunoassay on Roche Cobas e601 immunoassay analyzer (Roche Diagnostics, Germany). The recommended cutoff value of CA724 was less than 8.2 µ/mL. Levels below the cutoff value were defined as low while above the value as high. CA724 reduction ratio (%) = (1- posttreatment level/pretreatment level) ×100%.
Statistical analysis
The chi-squared test and multivariate logistic regression analysis were used to assess the relationship between ORR and clinical variables. To obtain a nearly normal distribution in the raw data, raw CA724 serum values were transformed into a natural logarithm. CA724 values before chemotherapy were compared with those after chemotherapy with the paired samples t-test. The independent sample t-test was applied to analyze the association of the decline ratio of CA724 with ORR. Receiver operating characteristic (ROC) curve was constructed to evaluate the role of CA724 reduction in predicting response to therapy. P<0.05 was considered as statistical significance. Statistical analyses were conducted by Stata version 12.0.
Results
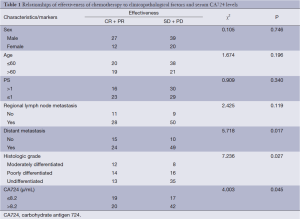
A total of 98 pathologically proven advanced GC patients were studied, and their characteristics are summarized in Table 1. There were 66 males and 32 females with a median age of 58 years (range, 35-70 years). The ORR was 39.80% with 2 CRs, 37 PRs, 39 SDs and 20 PDs. That was highly associated with the serum CA724 levels before chemotherapy; the difference in the ORR between CA724 high and low groups was significant (P=0.045). In addition, the ORR was also correlated with histologic grade (P=0.027) and distant metastasis (P=0.017). Table 2 indicates that by multivariate logistic regression analysis, the ORR was significantly associated with the pretreatment CA724 levels (OR =1.037; P=0.002; 95% CI, 1.013-1.061). There was also a marked association of the ORR with histologic grade (P=0.024) and distant metastasis (P=0.028).
Full table

Full table
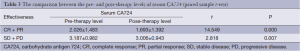
At the beginning of chemotherapy, 62 (63.27%) patients had serum CA724 levels above the cutoff limit, with a median value of 25.51 µ/mL (range, 0.10-75.00 µ/mL). After 2-6 cycles of chemotherapy, the overall median value dropped to 18.56 µ/mL (range, 0.08-124.01 µ/mL). Table 3 shows that the serum levels in different response groups declined significantly compared with baseline levels (P=0.000, and P=0.007, respectively; paired samples t-test). The mean reduction of CR + PR group and SD + PD group was 27.59% and –30.14%, respectively. There was a clear relationship between the reduction of CA724 and the ORR (T=2.059, P=0.044; independent sample t-test).
Full table
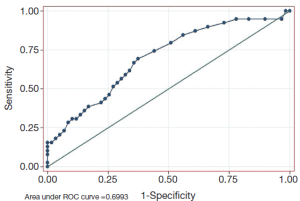
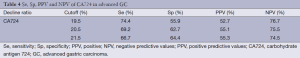
As shown in Figure 1, the decrease of CA724 in CR + PR group was related to the effectiveness of chemotherapy. The area under the ROC curve was 0.699 (P=0.001, 95% CI, 0.594-0.805). The sensitivity (Se), specificity (Sp), positive (PPV), and negative predictive values (NPV) of CA724 are listed in Table 4. The optimum cutoff point for CA724 was achieved at a 20.5% reduction, with an Se of 69.2%, and an Sp of 62.7%. CA724 response was observed in 22 of 59 patients who had no objective response and in 27 of 39 responders (χ2=9.583, P=0.002; chi-square test). The strong association between ORR and CA724 response was confirmed by means of logistic analysis (OR =0.211; P=0.002; 95% CI, 0.150-0.621).
Full table
Discussion
Globally, GC is still the fourth most common cancer and the second leading cause of cancer-related mortality (11,12). Even though techniques of chemoradiotherapy, molecular target therapy and surgery are improving, the median survival for advanced GC remains at 8-10 months, with less than 10% 5-year survival (13). Anti-cancer drugs are highly toxic and expensive, and the ORR of first-line palliative chemotherapy has been reported to be 30-45% in advanced GC patients (14). Imaging techniques to estimate the therapeutic effect is limited by time, cost and clinical experience, which will fail to detect the presence of small hepatic and peritoneal metastases and vascular involvement (15). Thus, the development of simple and economical tools would be fairly desired in the field of treatment monitoring, estimating prognosis and early prediction of response to therapy in order to optimize disease management on an individual basis. Tumor biomarkers are substances expressed in the process of tumorigenesis and progression that may indicate the presence of a neoplasm and/or reflect the relative malignancy burden and aggressive biology (16). Because of their easy and cost-effective measurement, tumor markers are expected to be potential factors for judging therapy efficacy and prognosis.
The European Society for Medical Oncology Clinical Recommendations and the National Comprehensive Cancer Network Clinical Practice for GC 2013 have not mentioned any tumor biomarker in the aspects of screening, diagnosis and follow-up monitoring (17,18). A wide range of serum biomarkers has been studied in connection with GC, in which more attention has been paid to CA724 (19,20). Aiming at Chinese population, a meta-analysis suggested that the single test of serum CA724 could do the best sensitivity and acceptable for the detection of GC (8). Higher serum concentrations of CA724 are positively related to TNM stage, recurrence, ascites, distant metastasis, risk of death and poor overall survival (21-23). Using logistic regression analysis, we found that ORR was clearly associated with the serum CA724 levels before chemotherapy, and the ORR in CA724 low group was significantly higher than that in CA724 high group. These facts may be explained by Yoon’s findings that in vitro chemosensitivity of GC patients was negative correlated with pretreatment CA724 levels (24). Additionally, the ORR was found in association with histologic grade and distant metastasis, which was a reflection of prognosis (25).
The above results all put emphasis on the diagnostic and prognostic value of CA724. No data to date are available about the relationship between changes of serum CA724 level and tumor response during chemotherapy, which plays an important role for patients with advanced GC. Based on patients with unresectable locally advanced or metastatic GC, the present study of multiple analyses concentrated on the influence of changes in serum CA724 values on clinical benefits. The subjects underwent an FLO regimen and the ORR was 39.80%, in accordance with previous research (26). The serum CA724 concentrations in different response groups fell significantly compared with baseline levels. ROC analysis demonstrated that patients who experienced a drop of 20.5% in serum levels of CA724 gained a better tumor response than the others.
Conclusions
In conclusion, changes in serum CA724 level may help to predict the sensitivity to chemotherapy. It might be of clinical significance for oncologists to evaluate chemotherapy efficacy earlier and conveniently. In the future, we will enlarge sample size to minimize bias and further identify its value.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Kanat O, O’Neil BH. Metastatic gastric cancer treatment: a little slow but worthy progress. Med Oncol 2013;30:464. [PubMed]
- Price TJ, Shapiro JD, Segelov E, et al. Management of advanced gastric cancer. Expert Rev Gastroenterol Hepatol 2012;6:199-208. [PubMed]
- Park SC, Chun HJ. Chemotherapy for advanced gastric cancer: review and update of current practices. Gut Liver 2013;7:385-93. [PubMed]
- Afaq A, Akin O. Imaging assessment of tumor response: past, present and future. Future Oncol 2011;7:669-77. [PubMed]
- Jo JC, Ryu MH, Koo DH, et al. Serum CA 19-9 as a prognostic factor in patients with metastatic gastric cancer. Asia Pac J Clin Oncol 2013;9:324-30. [PubMed]
- Chen XZ, Zhang WK, Yang K, et al. Correlation between serum CA724 and gastric cancer: multiple analyses based on Chinese population. Mol Biol Rep 2012;39:9031-9. [PubMed]
- Greene FL, Page DL, Fleming ID, et al. eds. AJCC cancer staging manual. 6th ed. New York: Springer, 2002.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [PubMed]
- Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2012: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 2012. [Epub ahead of print]. [PubMed]
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [PubMed]
- Power DG, Kelsen DP, Shah MA. Advanced gastric cancer--slow but steady progress. Cancer Treat Rev 2010;36:384-92. [PubMed]
- Chen XL, Chen XZ, Yang C, et al. Docetaxel, cisplatin and fluorouracil (DCF) regimen compared with non-taxane-containing palliative chemotherapy for gastric carcinoma: a systematic review and meta-analysis. PLoS One 2013;8:e60320. [PubMed]
- Luo G, Xiao Z, Long J, et al. CA125 is superior to CA19-9 in predicting the resectability of pancreatic cancer. J Gastrointest Surg 2013;17:2092-8. [PubMed]
- Kanakis G, Kaltsas G. Biochemical markers for gastroenteropancreatic neuroendocrine tumours (GEP-NETs). Best Pract Res Clin Gastroenterol 2012;26:791-802. [PubMed]
- Ajani JA, Bentrem DJ, Besh S, et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2013;11:531-46. [PubMed]
- Waddell T, Verheij M, Allum W, et al. Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi57-63. [PubMed]
- Nam DH, Lee YK, Park JC, et al. Prognostic value of early postoperative tumor marker response in gastric cancer. Ann Surg Oncol 2013;20:3905-11. [PubMed]
- Mittal A, Gupta SP, Jha DK, et al. Impact of various tumor markers in prognosis of gastric cancer. A hospital based study from tertiary care hospital of Kathmandu valley. Asian Pac J Cancer Prev 2013;14:1965-7. [PubMed]
- Li F, Li S, Wei L, et al. The correlation between pre-operative serum tumor markers and lymph node metastasis in gastric cancer patients undergoing curative treatment. Biomarkers 2013;18:632-7. [PubMed]
- Emoto S, Ishigami H, Yamashita H, et al. Clinical significance of CA125 and CA72-4 in gastric cancer with peritoneal dissemination. Gastric Cancer 2012;15:154-61. [PubMed]
- Liu X, Cai H, Wang Y. Prognostic significance of tumour markers in Chinese patients with gastric cancer. ANZ J Surg 2014;84:448-53. [PubMed]
- Yoon SN, Roh SA, Cho DH, et al. In vitro chemosensitivity of gastric adenocarcinomas to histone deacetylase inhibitors, compared to established drugs. Hepatogastroenterology 2010;57:657-62. [PubMed]
- Sougioultzis S, Syrios J, Xynos ID, et al. Palliative gastrectomy and other factors affecting overall survival in stage IV gastric adenocarcinoma patients receiving chemotherapy: a retrospective analysis. Eur J Surg Oncol 2011;37:312-8. [PubMed]
- Al-Batran SE, Hartmann JT, Probst S, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol 2008;26:1435-42. [PubMed]


