Internal compared with external drainage of pancreatic duct during pancreaticoduodenectomy: a retrospective study
Introduction
Pancreaticoduodenectomy is a standard treatment for various benign and malignant diseases of the pancreatic head and periampullary region. Operative mortality has significantly declined to less than 5% in the last decade, but the incidence of postoperative morbidity remains high at 40% to 50% (1,2). The most significant cause of morbidity and mortality after pancreaticoduodenectomy is development of postoperative pancreatic fistula (POPF), which occurs in 0 to over 30% of patients according to a recent review (3). Several pharmacological and technical interventions have been suggested to decrease the POPF rate, including placement of drainage in the pancreatic duct (4-6). However, the relative advantage of internal compared with external drainage is a controversial issue, and whether there is an associated decrease in the incidence of POPF is unknown.
The present retrospective study was performed to compare the incident rates of POPF after pancreaticoduodenectomy associated with internal or external drainage of the pancreatic duct.
Materials and methods
Patients
Between 1 January 1999 and 31 December 2011, 316 consecutive patients underwent pancreaticoduodenectomy with pancreatic duct drainage for benign or malignant pathologies of the pancreas or periampullary region at the Department of Hepatobiliary Surgery at Tianjin Third Central Hospital, China. An external drainage tube of the pancreatic duct was placed in 128 of these patients, while an internal drainage tube was placed in 188. The general condition of the patients was recorded.
Surgical technique
The pancreaticoduodenectomies were all performed by a team of surgeons specialized in pancreatic surgery. To achieve internal drainage, before reconstruction of the digestive tract, one end of a silicone catheter with multiple side-holes was inserted about 3 to 5 cm into the pancreatic duct, with the other end (about 20 cm) left in the intestinal lumen. External drainage was implemented by inserting one end of the catheter into the pancreatic duct and the other end punctured through the intestinal wall and leading out of the abdominal wall. The diameter of the catheter depended on the size of the pancreatic duct; the largest sized stent that could be passed into the pancreatic duct was used. Catheter migration was prevented by an anchoring stitch that secured the catheter onto the mucosa of the jejunal side of the pancreaticojejunostomy anastomosis using a single absorbable suture. Care was taken to ensure that there were no side-holes in the portion of the catheter in the jejunum. The digestive tract reconstruction was performed with Child’s technique, and the pancreaticoenterostomy by end-to-end invaginated anastomosis. One or two abdominal drainage tubes were placed around the pancreatic anastomosis.
Perioperative management
All the patients were infused to maintain water and electrolyte balance. Total parenteral nutrition was given to maintain the patients’ nutrition, and antibiotics were used to prevent infection. The amylase content of the abdominal drainage fluid was monitored every other day. Percutaneous peritoneal drainage will be performed if locational collection was founded in the abdominal cavity by ultrasound B or CT combined with abdominal infection symptoms or signs, and the amylase content of the drainage will also be tested. If there was no pancreatic fistula and the drainage was less than 20 mL, the external drainage catheter was removed 2-3 weeks after surgery. In cases of pancreatic fistula, the catheter was pulled out after the fistula healed.
Definitions
According to the International Study Group of Pancreatic Fistula (ISGPF), pancreatic fistula is defined as drain output of any measurable volume of fluid on or after postoperative day 3 with amylase content greater than three times the serum amylase activity. In this study, pancreatic fistulas were graded according to ISGPF criteria: grade A, clinically silent; grade B, requiring clinical intervention; and grade C, with severe clinical sequelae (7). Delayed gastric emptying was defined as the need for nasogastric decompression beyond the 10th postoperative day. Wound infection was the presence of pus requiring wound opening. Pulmonary infection was defined as a suggestive radiographic study with fever and requiring antibiotics. Perioperative mortality included intraoperative death, death within 30 d of surgery, and in-hospital death.
Statistical analysis
Statistical analyses were performed using SPSS software (version 19.0, SPSS Inc., Chicago, IL, USA). Categorical variables were analyzed using the chi-squared test and corrected chi-squared test, and continuous variables were evaluated by the unpaired, independent, two-tailed t-test. Continuous data were expressed a . P<0.05 was considered statistically significant.
Results
There were no significant differences between patients given external pancreatic duct drainage and those given internal with regard to gender, age, or comorbidities, including hypertension, coronary heart disease, diabetes, and cholangitis (Table 1). In addition, there were no differences in pancreatic duct diameter, pathological types, or preoperative levels of bilirubin or albumin (ALB) (Table 2).
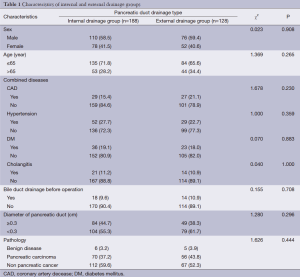
Full table
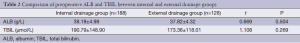
Full table
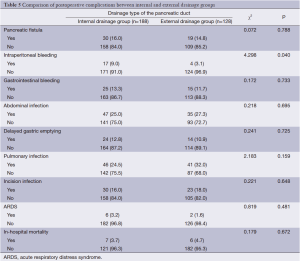
The three grades of fistula severity classified according to ISGPF clinical criteria were based solely on eight parameters (Table 3). A fistula was classified according to a particular grade if at least one criterion for that particular grade was present. Among the 316 patients, there were 49 cases of POPF. Thirty of these occurred in the internal drainage group (30/188, 16.0%) and 19 in the external drainage group (19/128, 14.8%). The incidence rates of POPF between the two groups were not statistically different. However, when the POPFs were stratified by grade, statistical differences were noted; POPFs in the internal drainage group were more severe than those of the external drainage group (P=0.014) (Table 4).
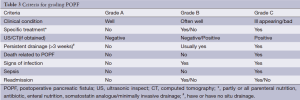
Full table
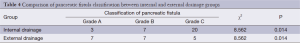
Full table
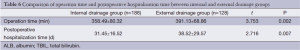
In addition, the rate of postoperative intraperitoneal bleeding was higher in the internal drainage group than the external drainage group (P=0.040). While there were no significant differences between the internal and external drainage groups regarding the occurrence of gastrointestinal bleeding, abdominal infection, pulmonary infection, acute respiratory distress syndrome (ARDS), or incision infection. There were six deaths in the external drainage group. These six patients died of intraperitoneal hemorrhage (n=1), intraperitoneal abscess (n=2), and pancreatic fistula (n=3), respectively. There were seven deaths in the internal drainage group. The causes of death were intraperitoneal abscess (n=2), pulmonary infection (n=1), gastrointestinal bleeding (n=1) and pancreatic fistula (n=3). There was no significant difference in the in-hospital mortality between the two groups (4.7% vs. 3.7%, P=0.672) (Table 5). Operative time and postoperative hospitalization of the external drainage group was longer than that of the internal drainage group (P=0.002 and P=0.007, respectively) (Table 6).
Full table
Full table
Discussion
POPF is one of the most frequent and serious complications after pancreaticoduodenectomy, and is often associated with the development of septic abdominal collections and hemorrhage that require reintervention (8-11). In recent years, many risk factors of pancreatic fistula after pancreaticoduodenectomy have been reported (3,12-14). These may be patient-related (age, gender, obesity, or basement disease) and may be combined with pancreatic factors such as pancreatic texture, pancreatic duct diameter, and histological type. In addition, there are factors related to the perioperative period (preoperative biliary drainage or chemotherapy, or postoperative prophylactic application of somatostatin or nutritional support) or related to the surgery itself (e.g., pancreatic anastomosis and pancreatic duct drainage placement). In the present study, the patients given internal drainage were not different than those given external drainage with regard to age, gender, comorbid basement disease, pancreatic duct diameter, pathological types, preoperative biliary drainage, postoperative prophylactic application of somatostatin, or postoperative nutritional support. Pancreaticoenterostomy of all the patients was by end-to-end sleeve anastomosis. Thus interference by the above risk factors on the results of the study was minimized.
We know that if gastrointestinal peristaltic function is not restored during the early period after pancreatoduodenectomy, then pancreatic juice and bile retention will occur in the initial segment of the jejunum (15,16). Trypsin will be activated when the pancreatic juice mixes with the bile, and the intestinal juice will have a strong corrosive effect leading to anastomosis. Digestive fluid retention in the jejunum can also increase pressure in the lumen and tension on the anastomosis. This significantly increases the probability of pancreatic fistula development (17,18).
Complete external drainage can avoid the corrosive effect of pancreatic fluid, and theoretically can reduce the occurrence of pancreatic fistula. A prospective randomized controlled study by Poon et al. (19) showed that external drainage of the pancreatic duct significantly reduced the incidence of POPF. Multicenter studies carried by Pessaux et al. (20) also suggested that external drainage of the pancreatic duct can significantly reduce the incidence of POPF and other complications in patients with relatively soft pancreatic texture and without pancreatic duct dilatation. However, external drainage of the pancreatic juice causes massive fluid and digestive enzyme lost, which may lead to delayed gastrointestinal function and physical recovery. To avoid secondary pancreatic fistula and extended hospitalization, the pancreatic duct drainage tube should only be pulled out 2-3 weeks after the sinus tract around the tube has completely formed.
Internal drainage of the pancreatic duct does not result in loss of pancreatic juice and digestive enzymes, and there is no need to wait 2-3 weeks to pull out the drainage tube. The question remains however whether internal drainage can reduce the incidence of pancreatic fistula after pancreaticoduodenectomy. According to studies by Winter et al. (21) and Smyrniotis et al. (22), internal drainage of the pancreatic duct could not reduce the incidence of POPF.
We found that although there was no statistical difference in the incidence of pancreatic fistula between the external and the internal drainage groups, the POPFs of the latter were of greater severity. Furthermore, the rates of postoperative intraperitoneal hemorrhage were higher in the internal drainage group than the external. We know that fistula most commonly occurs at the site of pancreatic-intestinal anastomosis, where pancreatic juice leaks into the abdomen. Biliary-intestinal anastomosis and gastrointestinal anastomosis are also susceptible to pancreatic fistula (23). In the internal drainage group of the present study, the drainage tube extending from the intestinal cavity was 10-20 cm long, which can cross the biliary intestinal anastomosis. However, due to postoperative dysfunction of intestinal motility, pancreatic-intestinal, biliary-intestinal, and gastrointestinal sutures were performed in the initial intestinal segments, delaying recovery of intestinal peristalsis even further and resulting in digestive fluid retention in this part of the intestine. Even when a short internal drainage tube is placed into the pancreatic duct, the pancreatic juice will not drain into the distal bowel over the gastrointestinal anastomosis. Thus, pancreatic enzymes mixed with bile and intestinal juice is promoted. Once a pancreatic fistula develops, pancreatic enzymes leak into the peritoneal cavity with great corrosive effect to peripheral tissues and blood vessels (24,25). Abdominal bleeding and infection secondary to the pancreatic fistula then correspondingly aggravate the severity of the fistula (26).
In addition, external drainage of the pancreatic duct can drain the majority of pancreatic juice to the in vitro, and though there may still some pancreatic juice discharging from the accessory pancreatic duct or pancreatic section, the volume of the pancreatic juice has been greatly reduced (27,28). Even if a pancreatic fistula occurs, the amount of pancreatic juice that may leak into the peritoneal cavity is not high, and the corrosive effect is greatly reduced. Therefore, abdominal bleeding associated with pancreatic fistula is reduced and the severity of pancreatic fistula is correspondingly lessened (29).
The present study is limited by its retrospective design. Because it was based on the review of medical records, it inevitably suffered from information bias. Furthermore, the study period was 13 years, which is too long to be optimal for the evaluation of postoperative complications.
Based on our analysis, we think that although external drainage of the pancreatic duct during pancreaticoduodenectomy cannot reduce the incidence of pancreatic fistula, it does significantly reduce the severity of pancreatic fistula. Our results show that the operative time and the postoperative hospitalization of the external drainage group were longer than those of the internal. Methods to decrease the operative time and hospitalization of patients provided with external drainage require investigation.
Acknowledgements
This study was supported by the Capital Health Research and Development of Special (No. 2011100203) and Science Foundation of Tianjin Health Bureau (No. 2013KZ011).
Disclosure: The authors declare no conflict of interest.
References
- Kawai M, Yamaue H. Analysis of clinical trials evaluating complications after pancreaticoduodenectomy: a new era of pancreatic surgery. Surg Today 2010;40:1011-7. [PubMed]
- Lai EC, Lau SH, Lau WY. Measures to prevent pancreatic fistula after pancreatoduodenectomy: a comprehensive review. Arch Surg 2009;144:1074-80. [PubMed]
- Ramacciato G, Mercantini P, Petrucciani N, et al. Risk factors of pancreatic fistula after pancreaticoduodenectomy: a collective review. Am Surg 2011;77:257-69. [PubMed]
- Kobayashi S, Gotohda N, Kato Y, et al. Infection control for prevention of pancreatic fistula after pancreaticoduodenectomy. Hepatogastroenterology 2013;60:876-82. [PubMed]
- Katsaragakis S, Larentzakis A, Panousopoulos SG, et al. A new pancreaticojejunostomy technique: a battle against postoperative pancreatic fistula. World J Gastroenterol 2013;19:4351-5. [PubMed]
- Tani M, Kawai M, Hirono S, et al. A prospective randomized controlled trial of internal versus external drainage with pancreaticojejunostomy for pancreaticoduodenectomy. Am J Surg 2010;199:759-64. [PubMed]
- Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8-13. [PubMed]
- Wronski M, Slodkowski M, Cebulski W, et al. Optimizing management of pancreaticopleural fistulas. World J Gastroenterol 2011;17:4696-703. [PubMed]
- Zhu F, Wang M, Wang X, et al. Modified technique of pancreaticogastrostomy for soft pancreas with two continuous hemstitch sutures: a single-center prospective study. J Gastrointest Surg 2013;17:1306-11. [PubMed]
- Roulin D, Cerantola Y, Demartines N, et al. Systematic review of delayed postoperative hemorrhage after pancreatic resection. J Gastrointest Surg 2011;15:1055-62. [PubMed]
- Kollmar O, Sperling J, Moussavian MR, et al. Delayed gastric emptying after pancreaticoduodenectomy: influence of the orthotopic technique of reconstruction and intestinal motilin receptor expression. J Gastrointest Surg 2011;15:1158-67. [PubMed]
- Callery MP, Pratt WB, Kent TS, et al. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg 2013;216:1-14. [PubMed]
- Miller BC, Christein JD, Behrman SW, et al. Assessing the impact of a fistula after a pancreaticoduodenectomy using the Post-operative Morbidity Index. HPB (Oxford) 2013;15:781-8. [PubMed]
- Ramacciato G, Mercantini P, Petrucciani N, et al. Risk factors of pancreatic fistula after pancreaticoduodenectomy: a collective review. Am Surg 2011;77:257-69. [PubMed]
- Qu H, Sun GR, Zhou SQ, et al. Clinical risk factors of delayed gastric emptying in patients after pancreaticoduodenectomy: a systematic review and meta-analysis. Eur J Surg Oncol 2013;39:213-23. [PubMed]
- Lermite E, Sommacale D, Piardi T, et al. Complications after pancreatic resection: diagnosis, prevention and management. Clin Res Hepatol Gastroenterol 2013;37:230-9. [PubMed]
- Uemura K, Murakami Y, Sudo T, et al. Elevation of urine trypsinogen 2 is an independent risk factor for pancreatic fistula after pancreaticoduodenectomy. Pancreas 2012;41:876-81. [PubMed]
- Yamashita S, Sakabe M, Ishizawa T, et al. Visualization of the leakage of pancreatic juice using a chymotrypsin-activated fluorescent probe. Br J Surg 2013;100:1220-8. [PubMed]
- Poon RT, Fan ST, Lo CM, et al. External drainage of pancreatic duct with a stent to reduce leakage rate of pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg 2007;246:425-33; discussion 433-5. [PubMed]
- Pessaux P, Sauvanet A, Mariette C, et al. External pancreatic duct stent decreases pancreatic fistula rate after pancreaticoduodenectomy: prospective multicenter randomized trial. Ann Surg 2011;253:879-85. [PubMed]
- Winter JM, Cameron JL, Campbell KA, et al. Does pancreatic duct stenting decrease the rate of pancreatic fistula following pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg 2006;10:1280-90; discussion 1290. [PubMed]
- Smyrniotis V, Arkadopoulos N, Kyriazi MA, et al. Does internal stenting of the pancreaticojejunostomy improve outcomes after pancreatoduodenectomy? A prospective study. Langenbecks Arch Surg 2010;395:195-200. [PubMed]
- Hashimoto Y, Traverso LW. Incidence of pancreatic anastomotic failure and delayed gastric emptying after pancreatoduodenectomy in 507 consecutive patients: use of a web-based calculator to improve homogeneity of definition. Surgery 2010;147:503-15. [PubMed]
- Oneil Machado N. Pancreatic fistula after pancreatectomy: definitions, risk factors, preventive measures, and management-review. Int J Surg Oncol 2012;2012:602478.
- Kimura W. Pancreaticojejunal anastomosis, using a stent tube, in pancreaticoduodenectomy. J Hepatobiliary Pancreat Surg 2009;16:305-9. [PubMed]
- Correa-Gallego C, Brennan MF, D’Angelica MI, et al. Contemporary experience with postpancreatectomy hemorrhage: results of 1,122 patients resected between 2006 and 2011. J Am Coll Surg 2012;215:616-21. [PubMed]
- Motoi F, Egawa S, Rikiyama T, et al. Randomized clinical trial of external stent drainage of the pancreatic duct to reduce postoperative pancreatic fistula after pancreaticojejunostomy. Br J Surg 2012;99:524-31. [PubMed]
- Kaman L, Nusrath S, Dahiya D, et al. External stenting of pancreaticojejunostomy anastomosis and pancreatic duct after pancreaticoduodenectomy. Updates Surg 2012;64:257-64. [PubMed]
- Rajarathinam G, Kannan DG, Vimalraj V, et al. Postpancreaticoduodenectomy haemorrhage: outcome prediction based on new ISGPS Clinical severity grading. HPB (Oxford) 2008;10:363-70. [PubMed]


