Clinical analysis of intracranial germinoma’s craniospinal irradiation using helical tomotherapy
Introduction
Germinoma is one of the common intracranial tumors in children. Most types of germinomas are sensitive to radiotherapy and chemotherapy. Patients in early stages can be cured for positive treatment and the 10-year survival rate is higher than 80%. However, intracranial germinomas mainly occur in pineal region, suprasellar region or basal ganglia region, and can easily spread through the cerebrospinal fluid (CSF). In addition to local recurrence, intramedullary implantation dissemination accounts for a large proportion of the failure cases. Craniospinal irradiation (CSI) has become the golden standard for the germinoma patients. It is very difficult to make a breakthrough in dose homogeneity and treatment accuracy of traditional radiotherapy methods as to the limitation of the length of the radiation field. As a brand-new radiotherapy technology, HT can easily solve the above-mentioned problems with its irradiation method and image guidance, and thus has been clinically recognized as one of the best choice for CSI. We retrospectively analyzed the clinical outcomes of HT for CSI on 23 patients.
Materials and methods
Clinical materials
From January 2008 to July 2012, 23 newly diagnosed intracranial germinoma patients treated with CSI using HT system in our center, including 18 males (78.3%) and 5 females (21.7%), with an average age of 20 (age range from 7 to 29). Nineteen of them were with single tumor, 5 in the pineal region, 8 in the sellar and pituitary region, and 6 on the one side of the basal ganglia region; for the remaining 4 cases, 2 on the both sides of the basal ganglia region, 1 in the sellar region and both of the cerebral ventricles, and 1 on the left side of the basal ganglia region and hypothalamus. The clinical symptoms and signs of the patients included diabetes insipidus (9 cases, 39.1%), dizziness and headache (5 cases, 21.3%), limb movement disorder (5 cases, 21.3%), visual impairment (4 cases, 17.4%), memory deterioration (3 cases, 13.0%), somnolence (2 cases, 8.7%), unexplained hematuria (1 case, 4.3%) and diabetic fatty liver (1 case, 4.3%). Six cases had been pathologically diagnosed, in which 5 cases were pure germinomas and 1 case was mixed germinomas; the remaining patients were clinically diagnosed by analyzing the imaging results as well as the β-human chorionic gonadotropin (β-HCG) and alpha-fetoprotein (AFP) levels in blood and CSF, or using the diagnostic radiotherapy methods (diagnostic radiotherapy with a dose of 20 Gy in the focal area followed by reexamination MRI indicates that the focus was gone or its size decreased by more than 50%) (1-3). Two and three cases had not blood β-HCG and AFP test before radiotherapy. Among the remaining patients, 6 cases (28.6%) and 1 case (5.0%) had high in blood β-HCG and AFP test, 4 cases (20%) had both high, and 10 cases (50%) had both normal. Fourteen patients (60.9%) had underwent CSF examination, in which 9 cases (64.3%) had high β-HCG level, and 1 case (4.3%) had tumor cells seen in the CSF smears. No distant metastasis was found in preoperative examinations (Table 1).
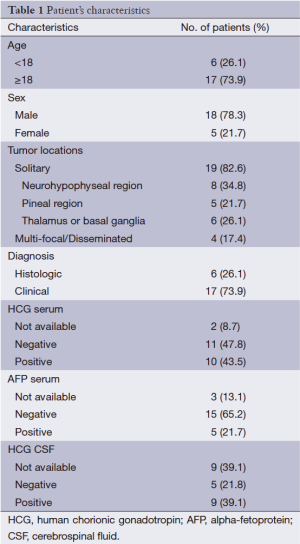
Full table
Radiotherapy
All patients were fixed in normal head and shoulder thermoplastic mask in supine position using an integrated board, Plain and enhanced CT images with 5-mm slice thickness were taken from overhead to tailbone for treatment planning using Philips spiral CT (Brilliance TM CT Big Bore) (Figure 1). The images were transmitted to Pinnacle3 8.0 workstation. Target delineation was based on patients’ enhanced MRI images or tumor positions seen in the surgery. Gross target volume (GTV) was defined as the primary tumors, residual tumors or tumor beds and pGTV was obtained by expanding the corresponding GTV with a margin of 5 mm. The craniospinal region was defined as clinical target volume (CTV), including the whole brain tissues, spinal canal, sacral region and sacral foramens, with an expansion of 5 mm defined as planning target volume (PTV). The PTV’s whole brain part includes the whole skull, with at least 3 mm away from the skin. For organs at risks (OARs), eyeballs, eye crystals, brain stem, lungs, heart, liver and kidneys were outlined. For female patients, their ovaries should be outlined as well. The prescribed doses were prescribed to cover at least 95% of the target volume. No more than 10% of pGTV and PTV volume received more than 110% of the prescribed doses, and no more than 1% of the volume received less than 93% of the prescribed doses. Fifteen patients including 13 patients underwent diagnostic radiotherapy were treated with local 3-dimensional conformal radiotherapy (3D-CRT) followed by CSI. Four patients were treated with CSI followed by 3DCRT. Three patients were treated with simultaneous integrated boost. All of the patients’ CSI used the HT system. The total doses were 27-36 Gy/15-20 F (1.5-2 Gy per fraction), and total local doses were 46-60 Gy/30-50 F (5 fractions per week). All female patients were treated with left-right parallel-opposed field irradiation (L5 vertebra or below) to protect their ovarian functions (DT 30-36 Gy/15-20 F, 1.8-2 Gy per fraction).
The CT images with the contour objects done by the physicians were transferred to Hi Art TomoTherapy 4.0.1 workstation. The same group of physicists designed and verified the treatment plans. Parameter settings could be set by the operator. A field dimension of 5.02 cm, a pitch of 0.287, a dose rate of 880 cGy/min and a modulation factor equal to 2.0 were used in our study (Figure 2).
All patients received daily image-guided radiotherapy (IGRT) by using megavoltage computed tomography (MVCT) of the HT system. Each patient was scanned twice, the skull and the body. The eyeballs should be avoided when scanning and the skull and the lower thoracic vertebrae were generally selected for body scanning. The thickness of a scanning slice was 4mm, and the length was 5-10 cm. Automatic and manual registration of the MVCT images with the planning CT images was based on bony and tissue anatomy and the micro-adjustment should be done if necessary. The longitudinal and vertical deviation should be adjusted by the average value for two calibration results and the lateral deviation was directly corrected twice.
Surgery and chemotherapy
Five patients underwent local tumorectomy, and 3 of them had their tumors partially cut. One patient had craniotomy for pathological biopsy. One patient underwent the third ventriculostomy and one patient had ventricular peritoneal shunt followed by local tumorectomy 6 months after radiotherapy. Seven patients had 2-6 cycles of chemotherapy, in which two cases underwent induced-chemotherapy, one case had concurrent chemotherapy, three cases had adjuvant chemotherapy, and one case had induced and adjuvant chemotherapy. The chemotherapy regimens were mainly include CE (carboplatin + VP-16), VMPP (cisplatin + methotrexate + vincristine + pingyangmycin) or the single drug temozolomide.
Follow-up and statistical analysis
During the radiotherapy, blood routine tests were reviewed weekly and acute side-effects were investigated and recorded. Patients reviewed follow-up examinations after the radiotherapy every 3 months for the first two years and semiannually two years later including blood routine tests, blood biochemistry, tumor marker, cerebral enhanced MRI, and spinal cord MRI if necessary. The follow-up time was calculated from the ending of radiotherapy. By the end of August 25th, 2013, the median follow-up was 30.9 months (range, 5-67 months). Survival analysis was performed by the method of Kaplan-Meier. The analyses were performed with the SPSS software package (Version 19.0, SPSS Inc., Chicago, IL, USA).
Results
Materials analysis
The average time to develop HT plans was 6 days (range, 4-10 days). The planing showed that, the average length for treatment-bed motion was 79.7 cm (range, 52.4-90.3 cm), and the average beam-on time was 722.2 s (range, 413.0-972 s). The mean dose (Dmean) of PTV was 32.4 Gy. The maximum dose of both lens was less than 5 Gy. The volume of both lungs receiving 10 Gy and 5 Gy (V10 and V5) were less than 10% and 40%, respectively. The V10 and V5 for liver were 4.7% and 40.9%, and the V10 and V5 for both kidneys were around 5% and 35% respectively (see Table 2).
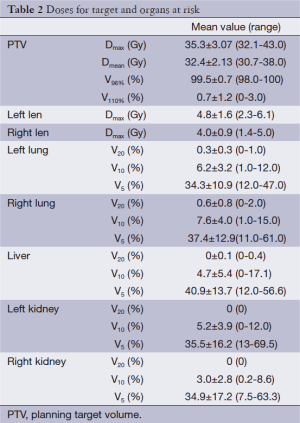
Full table
Acute and long-term toxic reactions
The acute and late side-effects were recorded according to the RTOG/EORTC standard. Hematological toxicity was one of the severest complications occurred in the procedure of CSI. The level 1-4 acute leukopenia were 8.7%, 30.4%, 34.8% and 21.7% and the level 1-4 acute thrombopenia were 8.7%, 30.4%, 21.7% and 8.7%, respectively. The anemia in this group occured by 26.1%, 13.0%, 8.7% and 0% (level 1-4) respectively. These hematologic toxicities were kept at relatively safe levels through drug intervention and most of the patients could finish the treatment except 1 case who suffered from both level 4 acute leukopenia and thrombopenia, stopped radiotherapy for two months and then completed the rest treatment. The patients’ hematologic indexs basically recovered to normal levels within 6 months after radiotherapy. 78.3% of the patients had nausea and vomiting at different levels. One patient could not tolerate the treatment because of severe gastrointestinal reactions at level 3 when the CSI was done only by 9 Gy/5 F, and then the plan was changed to whole brain radiotherapy. All patients’ hair began to grow in 1-3 months after the radiotherapy and reached their normal conditions in an average time of 6 months. The growth retardation was found in three children who were at the age of puberty (14-16 years old) and other adolescent patients grew samely as their peers. Abnormal hormone were found in two patients who had to take drugs to maintain their hormone levels.
Short-term effects and failure modes
The average follow-up time was 30.9 months (range, 5-67 months). According to the reexamination results one month after the radiation therapy, evaluation for primary lesions showed 9 (52.9%) complete responses (CR), 7 (41.2%) partial responses (PR), and 1 (5.9%) stable diseases (SD). Compared with that before treatment, the symptoms disappeared in 8 patients (38.1%), relieved in 10 (47.6%) patients, and no improved in 3 (14.3%) patients. In the group, 20 patients were free of disease. One 25-year old patient was verified tumor recurrence 8 months after the radiotherapy according to the reexamination images who received re-irradiation at the dose of 51 Gy/17 F with good tolerance, and was in good condition at present. Two patients (24 years old, male, and 29 years old, female) died of severely uncontrollable pulmonary infection in 3 months after radiation therapy, and they both suffered from severe myelosuppression during radiation therapy. The male patient who underwent concurrent chemoradiotherapy suffered from grade 4 leukopenia, grade 4 thrombopenia and grade 3 anemia. The female patient received radiotherapy alone suffered from grade 4 leukopenia, grade 3 thrombopenia and grade 3 anemia. Though they accomplished the treatment for the use of drugs, the haematological indexs still kept in low levers for a long while even after radiation therapy, for which they could be easily infected. In the group, the 3-year recurrence-free survival (RFS), distant disease-free survival (DFS) and overall survival (OS) were 95.2%, 100% and 91.3%, respectively.
Discussion
CSI for intracranial germinomas is clinically recognized as the most complicated radiotherapy method, which can be affected by many factors such as patient positions, fixing devices, placement repeatability and the accelerator’s radiation field length. Even if the conformal intensity-modulated radiotherapy (IMRT) which is relatively advanced is used, no guarantees can be made as to the treatment accuracy, efficiency and dose homogeneity. The HT system is a special type of dynamic IMRT system, which, unlike the normal accelerator, can complete its irradiation by rotating around the treatment couch at any angle (360°), and at the same time the treatment couch can go forward through the rack along its axis (4) to implement the “double movement” of the multi-leaf collimator and the treatment couch, in order to significantly improve the treatment efficiency and continuity without being affected by the sizes and shapes of the target areas. As a result, the MVCT image-guided system accompanied by the rack can also significantly correct the set-up errors and improve treatment accuracy, and thus it is clinically recognized as one of the preferred choices for CSI. Early in 2009, Sharma et al. (5) demonstrated that the HT for CSI had higher target area conformity and homogeneity than IMRT and 3DCRT, and at the same time, the maximum dose, average dose and accumulated dose for OARs are lower than IMRT and 3DCRT; Hong et al. (6) compared the HT plan and 3DCRT plan for three CSI patients and also reached the above-mentioned conclusions. In this study, PTV’s V96% reached 99.5%, and V110% reached only 0.7%. About OARs, the values for V20 in lungs were 0.3% and 0.6% respectively, while there was no 20 Gy radiation volume in liver and both kidneys.
Due to large irradiation scope, CSI will surely cause severe adverse reactions and hematological toxicity. This is also one of the key factors which determine whether or not a patient can successfully finish his/her therapy. Zaghloul et al. (7) used 3DCRT for CSI on 31 pediatric patients (7 years old on average) and found that 65% of the patients had gastrointestinal reactions such as nausea and vomiting, 10% of the patients had level 3 gastrointestinal reactions, 19% of the patients had hematological toxicity, and 55% of the patients had weight loss of more than 10%; Huang et al. (8) used CSI on 14 patients (5.8 years old on average, with 12 of them receiving 3DCRT and 2 of them receiving HT) and found that 21.4% of the patients had level 4 hematological toxicity. This symptom lasted for more than 5 weeks in one patient, and 3 patients were required to be admitted to the hospital for nutritional support. Similar situations also happened in the study of Sugie et al. (9) They used HT on 12 patients (14 years old on average) for CSI and found that 92% of the patients had level 3+ leukopenia, 42% of the patients had level 3+ thrombopenia and hemoglobin decrease. In this study, 78.3% of the patients had different levels of gastrointestinal reactions. And level 3+ leukopenia, thrombopenia and hemoglobin decrease were found in 56.5%, 30.4% and 8.7% of the patients respectively. It’s worth noting that, in the two studies mentioned above, all of the patients were pediatric patients, while in the third study, 6 (50%) of the patients were adult patients. Similarly, up to 60.9% of the patients were more than 20 years old in this group, and in five patients who had level 4 leukopenia, four patients were more than 23 years old (25.3 years old on average), and only one patient was pediatric patient. This seemingly demonstrates that adult patients are more likely to suffer from severe hematological toxicity than pediatric patients while receiving CSI. Currently, there isn’t too much clinical reports description on hair growth in patients receiving CSI. This study shows that all patients had hair growth within 1-3 months after finishing radiation therapy, and it took 6 months on average for them to reach normal quantity of hair completely. Clinical studies have already proved that radiotherapy can cause slow or sluggish bone tissue growth, especially in children at the age of puberty. Lower hormone secretion levels and developmental retardation of vertebral column may result in growth cessation and developmental malformation for the children receiving CSI. It can effect these children’s physical and psychological health as well as life qualities. There are no agreement on whether or not preventive hormone therapy can improve the above-mentioned conditions (10,11). In our study, we found 3 (13.0%) patients had growth cessation; no abnormal hormone levels were detected by hematological examination, and no hormone therapies were used to the patients in the follow-up. No developmental malformation was found in the patients,either. Kim et al. (12) gave 32 high-risk intracranial germinoma patients CSI therapies, and hormone level examinations were completed on 26 patients. The results show that abnormal hormone levels were detected in 38% of the patients; while in the study made by Cho et al. (13), the level of at least one hormone was found low in 54% of the patients, and the tumor locations in those patients were close to their pituitary glands. Because of local boost dosage, the irradiation doses received by the pituitary glands are higher than the prescribed doses received by the whole central system. In other word, any irradiation dosage less than 36 Gy has little effects on the functions of the pituitary glands. In this study, among 14 patients receiving hormone detection after the radiotherapy, 2 patients (29 years old and 11 years old) whose local tumors were in pituitary region were found low in hormone levels and need drug therapies.
Clinical studies had shown that the occurrence rate of acute radiation pneumonitis (ARP) was somewhat related to the average dose and low dosage volume of the exposed lung tissues. Schallenkamp et al. (14) studied 92 patients who received radiotherapy on their chests and found that the lung volume size receiving 10-15 Gy irradiation was directly related to ARP; whereas Wang et al. (15) studied the relationship between the esophagus cancer radiotherapy and ARP and found that the V5 volume size of the lung was more worth being considered. It was well known that the intensity-modulated arc therapy and volumetric modulated arc therapy (including the HT system) used in the clinic had obvious advantages in target area conformity and homogeneity as well as in high dosage areas of OARs, but at the same time the low dosage areas of many peripheral tissues were sacrificed. As a result, our center is always clinically cautious in considering the usage of this radiotherapy technology in patients who would receive radiotherapy on their chests, in order to lower the occurrence of ARP. In the studies of Peñagarícano et al. (16) and Sugie et al. (9), HT was used on 18 patients and 12 patients for CSI respectively, and 11 (61.1%) patients had their V10 >50% in the former study and the average V10 was 57.3% for the entire group of patients; the V10 and V5 for the entire lungs were 15.9% and 42.6% respectively in the latter study. No clinical ARP was found in the follow-ups for both studies. In this study, the V10 and V5 for left lung and right lung were 6.2%, 34.3%, and 7.6%, 37.4%, respectively, lower than the above-mentioned studies. However, it couldn’t be denied that, two patients in this group died of pneumonia infection within 3 months after their radiation therapy. It is known that ARP is usually related to irradiation of lungs, and infections are also essential factors, which are closely associated with autoimmunity and environment. Two died cases in this group both had level 4 myelosuppression during the radiotherapy even when they finished the treatment for a long time, the situation could not be effectively corrected, causing significantly higher opportunity of infection than other patients. From then on, our center had paid more attention to similar patients and strictly controlled the treatment indications, as well as actively corrected the side effects of radiotherapy. No above-mentioned situations happened again. Considering the low dosage feature of HT, this study used multiple-field irradiation for female patients, and the boundary was set at the L5 level. The whole brain, whole cervical cord and thoracic cord were irradiated by HT, and the sacrococcygeal region was irradiated by 3D-CRT. The ovary irradiation was strictly controlled in order to protect the patient fertility as much as possible. The specific impact will be observed in future’s follow-ups.
The prescribed doses used in this study were still traditional, with the CSI doses of DT 27-36 Gy/15-20 F (1.5-2 Gy per fraction), and total local doses of DT 46-60 Gy/30-50 F (5 fractions per week). The 3-year RFS, DFS and OS are 95.2%, 100% and 91.3% respectively, almost the same as reported by literatures. This dosage pattern had been clinically used for decades of years, and its side-effects were obvious. The therapy trend is to decrease dosage, combine with the chemotherapies, or decrease the target region in order to get better clinical results. Cho et al. (13) summarized their experiences of 31 years on CSI and demonstrated that CSI was still the standard therapy for intracranial germinomas. They found that all relapses occurred on patients receiving only local irradiation or whole brain irradiation, and it was safe and feasible to set prescribed dose as 19.5 Gy for CSI and 39.5 Gy for local irradiation. The famous multicenter, randomized SIOP CNS GCT 96 study (17) published their final results in 2013. This study showed that for local intracranial germinomas, using CSI for 24 Gy/15 F and local boost dosage to 40 Gy/25 F was the same result with the treatment using two cycles alternative chemotherapy of Carboplatin and Etoposide followed by local radiotherapy of 40 Gy/25 F instead of CSI. The 5-year event-free survival (EFS) and OS in two groups were 94% vs. 88% (P=0.13) and 95% vs. 96% (P=0.72) respectively, and there was no statistical significance. The 5-year progression-free survival (PFS) for the radiotherapy group was slightly better than the chemoradiotherapy group (97% vs. 88%, P=0.04). All of the failure cases were tumor recurrence in original sites or cerebral ventricles. This demonstrates that in theory, the combination of chemotherapy with local radiation can replace the traditional CSI if no proof of intramedullary dissemination is found. Currently, our center uses HT to implement CSI as a standard initial therapy for intracranial germinoma patients, and deliver 30 Gy for CSI and 50 Gy for local tumor as our standard dosage. At the same time, we also optionally choose chemoradiotherapy and decrease the radiotherapy dosage accordingly, in order to improve the patients’ treatment compliance and decrease the occurrence of late complications.
Conclusions
For primary intracranial germinomas, HT can be used to implement CSI for simplifying radiotherapy procedures, improving radiotherapy accuracy, enhancing protection of peripheral OARs and guaranteeing therapeutic effects. With the acceptable acute and long-term toxicity, CSI using HT in intracranial germinoma patients can be a safe and alternative mode.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Allen JC, Nisselbaum J, Epstein F, et al. Alphafetoprotein and human chorionic gonadotropin determination in cerebrospinal fluid. An aid to the diagnosis and management of intracranial germ-cell tumors. J Neurosurg 1979;51:368-74. [PubMed]
- Nakagawa K, Aoki Y, Akanuma A, et al. Radiation therapy of intracranial germ cell tumors with radiosensitivity assessment. Radiat Med 1992;10:55-61. [PubMed]
- Packer RJ, Cohen BH, Cooney K. Intracranial germ cell tumors. Oncologist 2000;5:312-20. [PubMed]
- Mackie TR, Holmes T, Swerdloff S, et al. Tomotherapy: a new concept for the delivery of dynamic conformal radiotherapy. Med Phys 1993;20:1709-19. [PubMed]
- Sharma DS, Gupta T, Jalali R, et al. High-precision radiotherapy for craniospinal irradiation: evaluation of three-dimensional conformal radiotherapy, intensity-modulated radiation therapy and helical TomoTherapy. Br J Radiol 2009;82:1000-9. [PubMed]
- Hong JY, Kim GW, Kim CU, et al. Supine linac treatment versus tomotherapy in craniospinal irradiation: planning comparison and dosimetric evaluation. Radiat Prot Dosimetry 2011;146:364-6. [PubMed]
- Zaghloul MS, Eldebawy E, Attalah E, et al. Supine craniospinal irradiation in children: patient position modification, dose uniformity and early adverse effects. Gulf J Oncolog 2012;(11):7-15.
- Huang F, Parker W, Freeman CR. Feasibility and early outcomes of supine-position craniospinal irradiation. Pediatr Blood Cancer 2010;54:322-5. [PubMed]
- Sugie C, Shibamoto Y, Ayakawa S, et al. Craniospinal irradiation using helical tomotherapy: evaluation of acute toxicity and dose distribution. Technol Cancer Res Treat 2011;10:187-95. [PubMed]
- Clayton PE, Shalet SM, Price DA. Growth response to growth hormone therapy following craniospinal irradiation. Eur J Pediatr 1988;147:597-601. [PubMed]
- Richards GE, Silverman BL, Winter RJ, et al. Dose dependency of time of onset of radiation-induced growth hormone deficiency. J Pediatr 1991;119:502-3. [PubMed]
- Kim JW, Kim WC, Cho JH, et al. A multimodal approach including craniospinal irradiation improves the treatment outcome of high-risk intracranial nongerminomatous germ cell tumors. Int J Radiat Oncol Biol Phys 2012;84:625-31. [PubMed]
- Cho J, Choi JU, Kim DS, et al. Low-dose craniospinal irradiation as a definitive treatment for intracranial germinoma. Radiother Oncol 2009;91:75-9. [PubMed]
- Schallenkamp JM, Miller RC, Brinkmann DH, et al. Incidence of radiation pneumonitis after thoracic irradiation: Dose-volume correlates. Int J Radiat Oncol Biol Phys 2007;67:410-6. [PubMed]
- Wang SL, Liao Z, Vaporciyan AA, et al. Investigation of clinical and dosimetric factors associated with postoperative pulmonary complications in esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys 2006;64:692-9. [PubMed]
- Peñagarícano J, Moros E, Corry P, et al. Pediatric craniospinal axis irradiation with helical tomotherapy: patient outcome and lack of acute pulmonary toxicity. Int J Radiat Oncol Biol Phys 2009;75:1155-61. [PubMed]
- Calaminus G, Kortmann R, Worch J, et al. SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol 2013;15:788-96. [PubMed]




