Breast cancer therapy by laser-induced Coulomb explosion of gold nanoparticles
Introduction
In recent years, interest in nanotechnology development has increased rapidly. One specific application of nanostructure is to enhance the treatment methods for breast cancer. From the breast cancer therapeutics point of view, nanostructures have become very useful tools for photothermal therapy due to enhanced absorption cross section (1,2). Such a strong absorption cross section ensures effective laser therapy at lower energies. Additionally, metal nanostructures such as gold and silver are photo-stable and do not suffer from photo-bleaching (3). However, among other inorganic nanoparticles, gold is the metal of choices for breast cancer treatment because of bio-modification and facile bio-conjugation. Currently, spherical gold nanoparticles are the main nanostructures that have been demonstrated in laser-induced photothermal therapy due to ease of conjugation to antibodies, photostability, lack of toxicity, and ease of fabrication (4). Many researchers believe that the laser-based thermotherapy with gold nanoparticles could be more effective than the current radiation-based treatments, with fewer adverse effects (5). It has been demonstrated that formation of gold nano-clusters on cell membranes increases the efficiency of bubble formation resulting in damage of breast cancer cells at a relatively low laser fluence of 3-5 mJ/cm2 (6). Significant and progressive approaches in the treatment of breast cancer have been realized in the last decade. Despite improvements in breast cancer treatment, the death rate among women over fifty years old is high because the malignant cells are resistant to existing therapies (7). Existing energy-based therapies can be classified into five categories: microwave ablation, cryoablation, radiofrequency ablation, high intensity focused ultrasound ablation and laser ablation (8). Compared to other techniques, laser-based therapy can be used as a protein denaturing agent in targeted cells. The photothermal therapies have a lot of advantages, such as less pain, effectiveness, low cost and capability of improving quality of life with less scaring and no incision. However, laser-based techniques tend to cause substantial damage to surrounding tissues. In the context of this background, breast cancer vestiges an inveterate disease by present treatment approaches. Cancers are assumed to arise from a succession of consecutive transmutations that take place due to chromosomal inconsistency (9,10). Although, the laser-irradiated gold nanoparticles have been used by several researchers (11-14), but there exists a great uncertainty regarding the killing of healthy cells. To clarify this problem, nanophotolysis approach for selective killing of malignant cells is applied. In previous studies, the nanophotolysis approach is adopted to selectively damage breast cancer cells (15,16).
In the present study, the gold nanoparticles with a radius of 50 nm could penetrate into tumor 1.22 cm in depth. Short laser pulse of 40 ns with 10 nm nanoaprticle radius could penetrate into tumor 1.14 cm in depth. Bubbles with a radius of 9 µm could kill breast cancer cells without damaging healthy cells.
Materials and methods
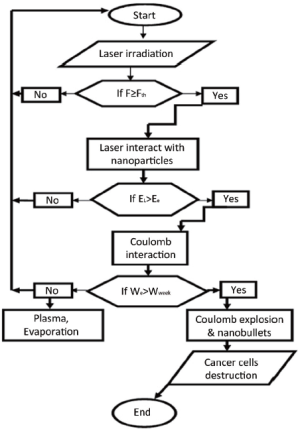
Flow chart (Figure 1) describes the way out of targeted therapy to breast cancer cells. Gold nanoparticles are irradiated by short pulse laser. When the laser fluence (F) is greater than the binding energy of gold atoms, electrons will be knocked out and the remaining ions will repel each other. The movement of electrons and ions depend on the absorbed energy during laser irradiation. If the energy of ions is sufficient to overcome the repulsive forces, ions in the form of nanobullets will strike on breast cancer and damage it completely.
Laser interaction with gold nanoparticles
The gold nanoparticles were irradiated with a short laser pulse of energy, EL(17).
[1] |
where (A) is the amplitude of wave, (ω) is the wave vector, and (z) is the propagating direction.
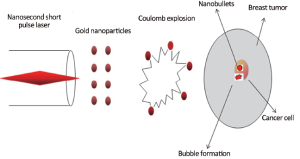
At a threshold (Fth) of laser fluence, the electrons come out from the surface and the remaining ions will exert repulsion, resulting in Coulomb explosion. Ions in the form of nanobullets emanate with a large pressure to hit the tumor and make a cluster of gold nanoparticles in the vicinity of the tumor cells. Penetration of nanopartilces into tumor and formation of cavitation bubbles are shown in Figure 2.
Penetration of gold nanobullets in tissues
Irradiance of laser in gold nanoparticles is described by the equation (17),
[2] |
Here, (f) is the laser fluence inside the medium (gold nanoparticles) at depth (z) of gold nanoparticles.
The irradiance (f0) is equal to the intensity (I0) of the incident radiation and accumulation factor (k) is the attenuation of the laser light intensity. Intensity is given by the penetration depth (δ) of tumor. Accumulation factor k is derived as a function of the diffuse reflectance (Rd) of laser light from gold surface.
[3] |
The diffuse reflectance (Rd) depends on the penetration depth (δ) and the absorption coefficient (µa),
[4] |
The penetration depth (δ) at which the irradiance decreases to can be calculated from (18),
[5] |
Here, is threshold laser intensity.
[6] |
Putting values of k in equation [5]
[7] |
Here (Fth) is the threshold laser fluence, (N) is the number of ions, (T) is the temperature, (Rp) is the radius of nanoparticles, (tp) is laser pulse duration, and (µ) is the absorption coefficient.
Spherical model of bubble dynamics
In order to discuss the surface tension, the liquid viscosity and compressibility of the liquid inside tumor, Brujan’s equation (a spherical model of bubble dynamics) is used, which calculates the bubble radius. The motion of bubble is defined as (19),
[8] |
Here, (H) is the change in enthalpy, (R) is the bubble radius, (C0) is sound speed in medium, and dot denotes time derivative. Enthalpy (H) is defined as,
[9] |
Here, (B) and (n) are constants obtained from the Tait equation to derive above equation (20). The pressure at the interface of bubble is given by,
[10] |
where (R0) is the bubble radius, k is the specific heat ratio at constant volume and pressure, P0 is the pressure inside the bubble, σ is the surface tension, and η is the viscosity.
Initial conditions are required to solve the equation [8]. An initial condition for can be obtained by putting the given initial condition for R and and in the incompressible limit C∞→ ∞. Assuming the laser energy absorbed by the gold nanoparticles during the laser ablation is used to increase the temperature of liquid surrounding the breast cancer, the relation of the bubble radius produced due to gold nanoparticles is given by,
[11] |
where (F) is the laser fluence (15 mJ/cm2), (Fth) is threshold laser fluence required to emanate gold ions from the gold surface, (ρcl) is the critical density (322 kg/m3) of liquid inside the tumor, (σabs) is the absorption cross section (2.93×10–15 m2) of gold nanoparticles of radius 60 nm, and (Ecl) is the internal energy (2,000 kJ/kg) of liquid.
Threshold fluence of laser is defined by (21),
[12] |
where (Rnp) is nanoparticles radius (30 nm), (Cnp) (0.13 kJ/kg-K), (ρnp) (19,300 kg/m3) and (T) (647 K) are the specific heat density, volume, and temperature at the critical point of liquid in tumor, respectively.
Solving equation [11] by using equation [12].
[12] |
Results
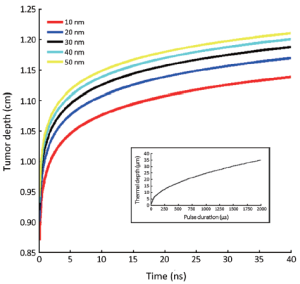
Figure 3 depicts the tumor depth as a function of the laser pulse duration. The nanosecond pulse durations were chosen to ionize the gold nanostructure and determine the threshold level for selective damage of abnormal cells in the breast cancer. For short pulse duration of 5-40 ns, penetration depth in tumor increases from 1.14 to 1.22 cm. The gold nanoparticles of 10, 20, 30, 40 and 50 nm in radius could penetrate into tumor 1.14, 1.155, 1.189, 1.20, and 1.22 cm in depth respectively. The maximum penetration depth in tumor can be obtained with nanoparticles of 50 nm radius. As the radius of nanoparticles increases, penetration of gold ions in the tumor goes on increasing. It is due to the increasing density of nanoparticle per unit volume.
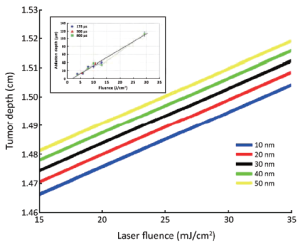
Laser irradiation by spherical gold nanoparticles of different radii with tumor depth is shown in Figure 4. The results showed that the increase in laser fluence causes penetration of gold ions in tumor depth. For 10 nm gold nanoparticles radius, the penetration depth of tumor is 1.504 cm. As the radius of particle decreases, tumor depth also increases. This is due to the large density of gold nanoparticles. Increase in the nanoparticle’s radius requires more energy from laser irradiation. Due to large radius, nanoparticles will go deep inside the tumor.
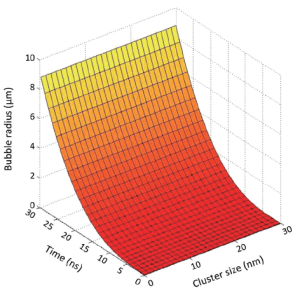
When a gold nanoparticles cluster of 10 nm radius is irradiated by laser pulse duration of 10 ns, a bubble with a radius of 4 µm is produced inside breast cancer. The bubble radius increases from 4 to 9 µm with an increase in pulse duration in the range of 10 to 30 ns as shown in Figure 5. Such an increase in the bubble radius may be sufficient to damage the cancerous cells with a radius of 8-13 µm. Bubble radius is greater than the size of cell radius because it loses energy as it goes inside the tumor. As the laser pulse duration goes beyond its maximum limit up to 30 ns, there is a linear increase in the size of bubble. It is due to the interaction of cavitations bubble with the malignant cells (22,23). These malignant cells absorb energy produced by bubble propagation. As the cluster size increases up to 30 nm, it causes an enhancement in the number of nanobullets.
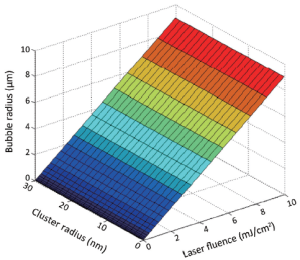
Results (Figure 6) showed that for a small laser fluence of 5 mJ/cm2, the laser interaction with a gold nanocluster of radius 10 nm causes an increase in surface tension of liquid in tumor. This produces contraction of vapors and therefore a bubble of smaller radius has no effect on the tumor cells of large size. Since the Coulomb repulsive forces between the gold ions are dominant over electrostatic forces of attraction, gold ions (nanobullets) will hit the tumor and produce a bubble of only 4 µm radius. Therefore laser fluence in this range is below the clinically useful threshold.
Discussion
For nanoparticles of 50 nm radius, the maximum penetration depth is obtained up to 1.22 cm (Figure 3) in the tumor which is the best fit for stage 2 or stage 3 breast cancer patients (23). Further increase in the pulse duration does not affect on tumor cells. This finding could be explained by that just few pulses of high laser energy for a short duration may kill the malignant cells via melting, fragmentation, and further increase in pulse duration may not make a sufficient damage. Our findings are in agreement to other experimental data (22).
As the particle radius increases up to 50 nm, tumor depth increases to 1.5199 cm in Figure 4 and similar kind of response is observed from other experimental findings (24). From these results, it can be concluded that the major damping mechanism during nanosecond short pulse irradiation is the liquid compressibility in the depth of the tumor.
The maximum number of ions (nanobullets) ejecting from the gold atom could produce bubble with a radius of 9 µm in breast cancer cells (Figure 5). Such size of bubble radius is comparable with the cell radius of 8-13 µm. Radius of gold nanoparticles in this range (30 nm) is utilized so that the healthy cells surrounding the tumor cells may not be affected. This bubble radius is suitable to enlarge the cell membrane to damage it.
Laser fluence at the maximum level of 15 mJ/cm2 hits the cluster size of radius 30 nm and produces the maximum number of nanobullets (Figure 6). These bullets hit the tumor and generate bubbles with a radius of 9 µm. Such fluence level and bubble size provide high selectivity of killing the breast cancer cells (24,25).
Selective nanophotolysis technique using gold nanoparticles and short laser pulse duration is theoretically investigated. Gold nanoparticles with radius of 10, 20, 30, 40, and 50 nm could penetrate into tumor 1.14, 1.155, 1.189, 1.20 and 1.22 cm in depth respectively. Short laser pulse of 40 ns with 10 nm nanoaprticle radius could penetrate into tumor 1.14 cm in depth. Bubble with a radius of 9 µm can effectively kill the breast cancer cells without damaging the healthy ones. Gold nanoparticles with increasing radius and bubble formation for selective damaging of breast cancer cells are successfully probed. It is possible to control the extent of cellular injury in a tumor volume by controlling the size of bubbles. In this mode, gold nanoparticles of 10-50 nm radius and bubble with a radius of 9 µm can effectively kill the breast cancer cells without damaging the surrounding healthy tissues.
Acknowledgements
The authors would like to thank for the financial support by Fund of the Ministry of Higher Education (MOHE) Malaysia and Universiti Teknologi Malaysia (UTM) Skudai, Johor, Malaysia under Grant No Q.J130000.2526.02H93/-03H78.
Disclosure: The authors declare no conflict of interest.
References
- Pattani VP, Tunnell JW. Nanoparticle-mediated photothermal therapy: A comparative study of heating for different particle types. Lasers Surg Med 2012;44:675-84. [PubMed]
- Huang X, Jain PK, El-Sayed IH, et al. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci 2008;23:217-28. [PubMed]
- Yu MK, Park J, Jon S. Targeting strategies for multifunctional nanoparticles in cancer Imaging and therapy. Theranostics 2012;2:3-44. [PubMed]
- Zhao Z, Wu F. Minimally-invasive thermal ablation of early-stage breast cancer: A systemic review. Eur J Surg Oncol 2010;36:1149-55. [PubMed]
- Letfullin RR, Joenathen C, George TF. Laser-induced explosion of gold nanoparticles: potential role for nanophotothermolysis of cancer. Nanomedicine (Lond) 2006;1:473-80. [PubMed]
- Zharov VP, Galitovsky V, Viegas M. Photothermal guidance of selective photo-thrmolysis with nanoparticles. Proc SPIE 2004;7:291-300.
- Pustovalov V, Zahrov V. Threshold parameters of selective nanophothermolysis with Gold nanoparticles. Int. Conference BIOS 2008. San-Jose. 2008. USA. Proc. SPIE 2008;6854:39.
- Sun JM, Gerstman BS, Li B. Bubble dynamics and shock waves generated by laser absorption of a photoacoustic sphere. J Appl Phys 2000;13:2352-62.
- Marsh M, Schelew E, Wolf S, et al. Gold nanoparticles for cancer treatment. PHYS 483 – Queen’s University, Kingston, March 29, 2009.
- Al-Hajj M, Wicha MS, Benito-Hernandez A. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003;100:3983-8. [PubMed]
- Ashiq MGB, Saeed MA. Laser induced Coulomb explosion of gold nanoparticles: application of nanophotolysis for Breast cancer. J Int Pulse laser Appl Adv Phys 2012;2:1-3.
- Zharov VP, Letfullin RR, Galitovskaya EN. Microbubbles-overlapping mode for laser killing of cancer cells with absorbing nanoparticle clusters. J Phys D: Appl Phys 2005;38:2571-81.
- Huang X, Qian W, El-Sayed IH. The potential use of the enhanced nonlinear properties of gold nanospheres in photothermal cancer therapy. Lasers Surg Med 2007;39:747-53. [PubMed]
- Cianfrocca M, Goldstein L J. Prognostic and predictive factors in early-stage breast cancer Oncologist 2004;9:606-16. [PubMed]
- Ashiq MGB, Ibrahim N, Shahid M, et al. Novel nanophotolysis technique for breast cancer therapy. Modern Physics Letters B 2012;26:1250147-55.
- Ashiq MGB, Saeed NA, Ibrahim N, et al. Numerical study of nanophotolysis approach for breast cancer. Modern Physics Letters B 2012;26:1250187-94.
- Annou R, Tripathi VK. Femtosecond laser pulse induced coulomb explosion. 34th EPS Conf. Plasma Phys 2007;31F:5,108.
- Barton TG, Foth HJ, Christ M, et al. Interaction of holmium laser radiation and cortical bone: ablation and thermal damage in a turbid medium. Appl Opt 1997;36:32-43. [PubMed]
- Brujan EA. Numerical investigation on the dynamics of cavitation nanobubbles. Microfluid Nanofluid 2011;1:511-17.
- Tait PG. eds. Physics and Chemistry of the Voyage of HMS Challenger. London: HMSO, 1888.
- Letfullin RR, George TF, Duree GC. Ultra short laser pulse heating of nanoparticles: comparison of theoretical approaches. Adv Opt Technol 2008;1:450-58.
- Matjaz L, Perhavec T, Nemes K, et al. Ablation and thermal depths in VSP Er:YAG laser skin resurfacing. J Laser Health Acad 2010;1:56-71.
- Zharov VP, Galitovsky V, Viegas M. Photothermal detection of local thermal effects during selective nanophotothermolysis. Appl Phy Lett 2003;83:4897-99.
- Letfullin RR, Joenathan C, George TF, et al. Laser-induced thermal explosion of nanoparticles: potential role for nanaophotothermolysis of cancer. Nanomedicine (Lond) 2006;1:473-80. [PubMed]
- Pustovalov VK. Theoretical study of heating of spherical nanoparticle in media by short laser pulses. Chemical Physics 2005;30:103-8.