Prognostic relevance of number and ratio of metastatic lymph nodes in resected carcinoma of the ampulla of Vater
The ampulla of Vater is located on the inner or posterior wall of the descending duodenum in most people. It is formed by the union of the pancreatic duct and the common bile duct, with the duodenal papilla (or, the Vater papilla) as the opening. The cancer can occur inside the ampulla or on the papilla, and the tumor tissue can arise from the pancreatic duct epithelium, bile duct epithelium, or duodenal mucosa epithelium. Compared with pancreatic cancer and distal cholangiocarcinoma, the Carcinoma of the ampulla of Vater (CAV) responds better to surgical resection. Currently, the standard surgical procedure for CAV is pancreaticoduodenectomy (PD), although the range of lymph node dissection during it remains undefined. Due to its low incidence, the case numbers were low in most reports. In our current study, the reported case number in the past two decades was relatively large, reaching 155. Recent literature has shown that the number of metastatic lymph nodes and the lymph node ratio (LNR) are factors that affect the prognosis of gastrointestinal (GI) malignancies (1,2). In particular, they are two important prognostic factors for both pancreatic cancer and for colorectal cancer (3,4). However, for CAV, few studies have been conducted on this regard. In fact, further research on the prognosis of CAV in terms of pathologic factors as well as the status and locations of lymph node metastasis will be valuable for the selection of surgical procedures and adjuvant therapy. In our current study, by investigating the lymph node involvement in the resected CAV and the potential prognostic factors, we tried to identify the features of lymph node metastasis and prognosis in CAV and thus select the effective surgical treatment for this disease.
Methods
General data
A total of 155 patients with pathologically confirmed CAV who underwent radical resection between January 1990 and December 2010 were enrolled in this study. Among them there were 89 men and 66 women, aged 29-78 years (median: 55 years). Of these patients, 123 (79.4%) were presented due to jaundice. Tumor marker CA 19-9 was positive in 75 (60%) of 125 patients, and CEA was positive in 25 (18.52%) of 135 patients.
Surgery and pathology
The standard PD was performed in all the 155 patients with CAV. The majority of the stomach, part of the omentum, head of the pancreas, duodenum, upper jejunum, lower common bile duct, and regional lymph nodes were removed. Gallbladder was preserved in some patients. If swollen lymph nodes were detected near the superior mesenteric artery and/or abdominal aorta, selective dissection was performed. Tissue samples were reviewed by two pathologists in our hospital. All patients underwent an R0 resection, and the pathological staging was based on the 7th edition of the AJCC cancer staging manual. The histological types included 154 cases of adenocarcinoma and one case of adenosquamous carcinoma.
Statistical methods
The relationship between clinicopathological parameters and lymph node metastasis was analyzed by chi-square test, and the survival rates were calculated using the Kaplan-Meier method. All the statistical analyses were conducted using the SPSS statistical software (version 19.0 IBM SPSS Inc., Chicago, IL, USA). Kaplan-Meier method was applied for the univariate analysis of the prognosis. Multivariate analysis of the potential prognostic indicators was performed by the Cox proportional hazards stepwise regression model.
Complications
Among these 155 patients with CAV, 7 patients died postoperatively, yielding an in-hospital mortality rate of 4.5%. Of these 7 patients, 4 died of multi-organ failure, 1 suicided, and 2 died of postoperative bleeding/shock. Postoperative complications were reported in 66 cases (42.6%), including anastomotic leakage/bile leakage/pancreatic leakage (n=24, 15.4%), delayed gastric emptying (n=16, 10.3%), GI bleeding (n=15, 9.7%), lung infections (n=7, 4.5%), and abdominal infections (n=4, 2.6%). The complications were cured after conservative treatment or surgical treatment.
Results
Number of metastatic lymph nodes in the resected CAV
In these 155 patients with CAV, lymph node involvement was pathologically detected in 33 patients (21.3%). A total of 1,524 lymph nodes were detected, with a median of 8.00. Totally 82 positive lymph nodes were found. The locations (and their frequencies) of the metastatic lymph nodes included: around the head of the pancreas 75.8% (25/33); intestinal wall, 18.2% (6/33); pyloric region, 3.1% (1/33); and near the gut, pancreas, and bile duct, 3.1% (1/33).
Among them, 16, 6, 5, and 7 patients had 1, 2, 3, and 4 metastatic lymph nodes, respectively. The regions of the involved solitary lymph node metastasis in 16 patients with CAV included: around pancreatic head (n=11), intestinal wall (n=4), and common bile duct (n=1). Obviously, the majority of the solitary lymph node metastasis located around the head pancreatic head (68.8%).
Of the 155 cases of CAV analyzed, without lymph metastasis seen in 121 cases, solitary lymph node metastasis in 16 cases. The patients’ sex, age, medical history, jaundice, tumor diameter, histological type, tumor differentiation, T-stage, TNM stage, depth of duodenal invasion, depth of pancreatic invasion and other factors were input into the computer database. Chi-square test showed that the following factors were related with the solitary lymph node metastasis: depth of duodenal involvement (P=0.037), depth of pancreatic invasion (P=0.033), T-stage (P=0.030) and TNM stage (P=0.000) (Table 1).

Full table
Ratio of metastatic lymph nodes in the resected CAV
The LNR was calculated by the number of the metastatic lymph nodes over the detected lymph nodes. It was divided into three groups: 0, 0-20%, >20%, which accounted for 78.1% (121/155), 9.7% (15/155), and 12.3% (19/155), respectively.
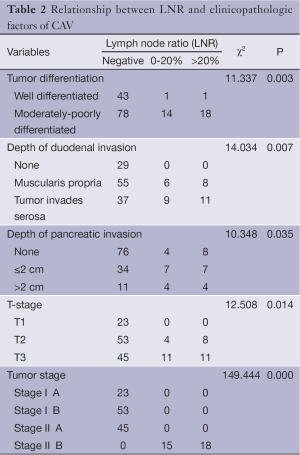
The patients’ sex, age, medical history, jaundice, tumor diameter, histological type, tumor differentiation, T-stage, TNM stage, depth of duodenal invasion, depth of pancreatic invasion and other factors were input into the computer database. Chi-square test showed that the following factors were related with the LNR: tumor differentiation (P=0.003), depth of duodenal involvement (P=0.007), depth of pancreatic invasion (P=0.035), T-stage (P=0.014) and TNM-Stage (P=0.000) (Table 2).
Full table
Clinicopathologic factors associated with lymph node metastasis
The patients’ sex, age, medical history, jaundice, tumor diameter, histological type, tumor differentiation, T-stage, TNM stage, number of lymph nodes, number of metastatic lymph nodes, depth of duodenal invasion, depth of pancreatic invasion and other factors were input into the computer database. Chi-square test showed that the following factors were related with the lymph node metastasis: tumor differentiation (P=0.001), depth of duodenal involvement (P=0.001), depth of pancreatic invasion (P=0.005), T-stage (P=0.002) and jaundice (P=0.016) (Table 3).
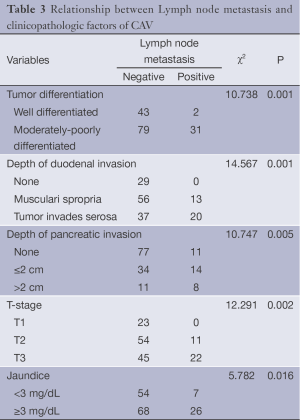
Full table
Univariate and multivariate analyses on the prognostic factors
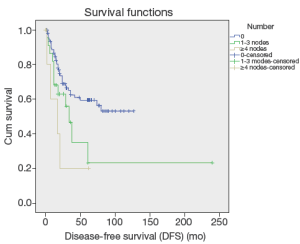
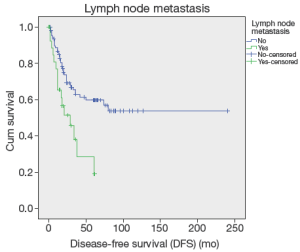
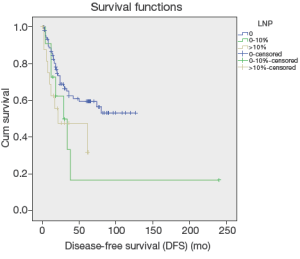
Up to March 2012, the follow-up rate reached 91%. The 5-year cumulative survival was 51.6%, and the 5-year disease-free survival rate was 54.6%. Univariate analysis showed that the factors associated with the prognosis included: tumor size (P=0.036), tumor differentiation (P=0.019), number of metastatic lymph nodes (P=0.024) (Figure 1), lymph node metastasis (P=0.03) (Figure 2), LNR (P=0.032) (Figure 3), depth of pancreatic invasion (P= 0.001), T-stage (P=0.002), TNM stage (P=0.001), elevated CA 19-9 (P=0.000), and jaundice (P=0.021) (Table 4). Multivariate analysis showed that the factors associated with the prognosis were the number of metastatic lymph nodes (P=0.032; RR: 1.283; 95% CI: 1.022-1.611), tumor size (P=0.043; RR: 1.736; 95% CI: 1.017-2.963), and elevated CA 19-9 (P=0.003; RR: 3.247; 95% CI: 1.504 -7.010).
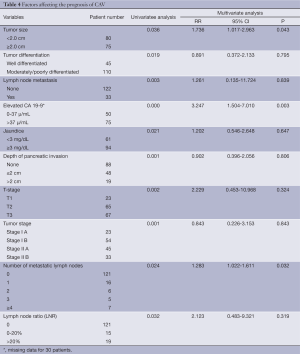
Full table
Discussion
The ampulla of Vater is a special site of anatomy. The incidence of tumors occur in this region is low, accounting about 0.2% among all the GI carcinomas (5). Due to its low incidence, the case numbers were low in most reports. As a result, the risk factors and prognoses of this disease remarkably differed in literature, with a 5-years survival rates ranging from 36.8-75.2% (6-10). Similarly, the 5-year survival rate in patients who had undergone radical surgical resection was 51.6% in our study. The prognostic factors found in our study were: diameter and differentiation of tumors, LNR, number of metastatic lymph nodes, pancreatic involvement, T stage, TNM stages, serum level of CA 19-9, and appearance of jaundice. Among them, the most important factors are the number of metastatic lymph nodes, tumor size, and elevated CA 19-9. Similar to our report, a variety of prognostic factors associated with five years survival rate have been reported, such as jaundice, transfusion during surgery, lymph node metastasis, tumor differentiation, tumor size, and clinical staging, and positive surgical margin. Although the conclusion in different reports varied much, lymph node metastasis is common in all of reports, as the most important prognostic factor of the CAV (6,10-14). However, LNR of the CAV in reports varied very dramatically, ranging from 29-52% (15-17). In our study, the lymph node positive disease of 155 cases with the CAV was 21.3%, lower than that in previous reports. Low lymph node positive disease in our study was mainly caused by more cases with jaundice and early-stage tumor that was determined after surgery. Recent studies showed that location, number and rate of lymph node metastasis remarkably affected the prognosis of pancreatic cancer. Similar findings were also reported in studies of other GI carcinomas (2,3). Therefore, study on the location, number, and rate of lymph node metastasis is of important clinical significance.
The number of the metastatic lymph nodes is one of the most important prognostic factors of the CAV. In our current study, the 5-year survivals of patients with different numbers of metastatic lymph nodes (0, 1, or more than 2) were 46.6%, 28.4%, 0%, which showed statistical significances in both the univariate and multivariate analyses (all P<0.05), implicating that lymph node metastasis significantly affects the prognosis of patients after the radical surgery of the CAV. De Castro et al. also demonstrated the role of single lymph node metastasis in predicting the prognosis (16). A sentinel lymph node (SLN) is the first lymph node(s) associated with tumor lymphatic drainage, to which cancer cells are most likely to spread from a primary tumor (18). Theoretically, if SLN is not involved, the other lymph nodes might be safe. According to concept of SLN, solitary lymph node metastasis is commonly referred as to involvement of SLN (19). The regions of the involved SLN in this study included: around pancreatic head (n=11), intestinal wall (n=4), and common bile duct (n=1). SLN involvement in the region around pancreatic head accounted for 68.8%, indicating that 68.8% (11/33) solitary lymph node metastasis occurred in the pancreatic head-duodenal region. According to the standard of SLN for pancreatic cancer, which was made by Japan Pancreas Society (20), only six patients with the ampulla of Vater were found with skip metastasis, implicating the metastatic pathway of the CAV following the law from near to far. Those findings are informative and helpful to dissection of metastatic lymph nodes or sentinel lymph node biopsy in patients with surgery of either local resection or pseudocapsule dissection. Overall, the affecting factors for lymph node metastasis in the ampulla of Vater include depth of tumor invasion, tumor size, differentiation of tumor, involvement of pancreas, and nerve involvement and so forth (1-4,13,15,17).
Depth of tumor invasion was reported in literature as an important affecting factor for lymphatic metastasis in the CAV. Risk of lymphatic metastasis is promoted with an increase in depth of tumor invasion, leading to poor prognosis of patients with the CAV (2-4,11,13,21-23). Metastatic lymph nodes in the CAV are mainly located in the region surrounding the head of pancreas (13th and17th station), but lymph nodes in other stations are less involved (2-4). Consistent with other reports, our study on the location of metastatic lymph nodes also found that degree of tumor differentiation, involvement of duodenum and pancreas, T-staging and jaundice are affecting factors for the CAV. Similar to previously reports (2-4), our results showed the distribution of metastatic lymph nodes in the CAV: around pancreatic head 75.8% (25/33), intestine 18.2% (6/33), pyloric region 3.1% (1/33), metastasis of intestinal lymph nodes, parapancreatic lymph nodes, peribiliary lymph nodes 3.1% (1/33). Our results showed that more than ampullary cancer lymph node metastasis in pancreatic around. Similar findings in a study on 52 cases of the CAV was reported by Lee et al. (2). Therefore, radical lymph node dissection is required for the cases with metastatic lymph nodes surrounding pancreatic head (13th and17th station) and more deeply tumor invasion. The LNR is another affecting factor for the prognosis of patients with GI malignancies. LNR is defined as the ratio of the number of positive lymph nodes to the number of removed lymph nodes, of which calculation may minimize the difference between clinical evaluation of prognosis and the real status of the lymph nodes. LNR is commonly used for the prognosis of patients with pancreatic and colorectal cancer (24,25), but it is rarely reported for the prognosis of patients with the CAV, due to the low incidence of this disease. Lee et al. (2) reported a study on 52 cases with lymphatic metastasis in the CAV, in which LNR was identified by univariate analysis, but not by multivariate analysis as an independent prognostic factor. A similar study with a significant LNR (P<0.0001) was reported by Sakata et al. (1), which had 71 cases of the CAV. In our study, 33 cases with lymphatic metastasis were used for univariate analysis, which revealed significantly difference between LNR of primary tumor and 5-year disease-free survival rate (P<0.05), consistent with the conclusions of other reports as mentioned above. Furthermore, a recent study reported by Christina on 143 patients with the CAV (4), which also confirmed LNR is an independent prognostic factor. It is obvious that more scholars’ attention has been paid to LNR as the prognostic factor for patients with the CAV. We believe, further studies on LNR with accumulated cases will improve its accuracy in the assessments of prognosis and treatment for patients with lymphatic metastasis.
Conclusions
The LNR is a useful factor for predicting the prognosis of the radical treatment for CAV, whereas the number of metastatic lymph nodes is the most important factor. Further research on the locations, number, and LNR will be clinically meaningful to improve survival in patients with CAV.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Sakata J, Shirai Y, Wakai T, et al. Assessment of the nodal status in ampullary carcinoma: the number of positive lymph nodes versus the lymph node ratio. World J Surg 2011;35:2118-24. [PubMed]
- Lee JH, Lee KG, Ha TK, et al. Pattern analysis of lymph node metastasis and the prognostic importance of number of metastatic nodes in ampullary adenocarcinoma. Am Surg 2011;77:322-9. [PubMed]
- Pomianowska E, Westgaard A, Mathisen Ø, et al. Prognostic relevance of number and ratio of metastatic lymph nodes in resected pancreatic, ampullary, and distal bile duct carcinomas. Ann Surg Oncol 2013;20:233-41. [PubMed]
- Roland CL, Katz MH, Gonzalez GM, et al. A high positive lymph node ratio is associated with distant recurrence after surgical resection of ampullary carcinoma. J Gastrointest Surg 2012;16:2056-63. [PubMed]
- Neugut AI, Jacobson JS, Rella VA. Prevalence and incidence of colorectal adenomas and cancer in asymptomatic persons. Gastrointest Endosc Clin N Am 1997;7:387-99. [PubMed]
- Barauskas G, Gulbinas A, Pranys D, et al. Tumor-related factors and patient’s age influence survival after resection for ampullary adenocarcinoma. J Hepatobiliary Pancreat Surg 2008;15:423-8. [PubMed]
- Brown KM, Tompkins AJ, Yong S, et al. Pancreaticoduodenectomy is curative in the majority of patients with node-negative ampullary cancer. Arch Surg 2005;140:529-32; discussion 532-3. [PubMed]
- Di Giorgio A, Alfieri S, Rotondi F, et al. Pancreatoduodenectomy for tumors of Vater’s ampulla: report on 94 consecutive patients. World J Surg 2005;29:513-8. [PubMed]
- O’Connell JB, Maggard MA, Manunga J Jr, et al. Survival after resection of ampullary carcinoma: a national population-based study. Ann Surg Oncol 2008;15:1820-7. [PubMed]
- Yoon YS, Kim SW, Park SJ, et al. Clinicopathologic analysis of early ampullary cancers with a focus on the feasibility of ampullectomy. Ann Surg 2005;242:92-100. [PubMed]
- Hatzaras I, George N, Muscarella P, et al. Predictors of survival in periampullary cancers following pancreaticoduodenectomy. Ann Surg Oncol 2010;17:991-7. [PubMed]
- Duffy JP, Hines OJ, Liu JH, et al. Improved survival for adenocarcinoma of the ampulla of Vater: fifty-five consecutive resections. Arch Surg 2003;138:941-8; discussion 948-50. [PubMed]
- de Paiva Haddad LB, Patzina RA, Penteado S, et al. Lymph node involvement and not the histophatologic subtype is correlated with outcome after resection of adenocarcinoma of the ampulla of vater. J Gastrointest Surg 2010;14:719-28. [PubMed]
- Qiao QL, Zhao YG, Ye ML, et al. Carcinoma of the ampulla of Vater: factors influencing long-term survival of 127 patients with resection. World J Surg 2007;31:137-43; discussion 144-6. [PubMed]
- Winter JM, Cameron JL, Olino K, et al. Clinicopathologic analysis of ampullary neoplasms in 450 patients: implications for surgical strategy and long-term prognosis. J Gastrointest Surg 2010;14:379-87. [PubMed]
- de Castro SM, van Heek NT, Kuhlmann KF, et al. Surgical management of neoplasms of the ampulla of Vater: local resection or pancreatoduodenectomy and prognostic factors for survival. Surgery 2004;136:994-1002. [PubMed]
- Heinrich S, Clavien PA. Ampullary cancer. Curr Opin Gastroenterol 2010;26:280-5. [PubMed]
- Cabanas RM. An approach for the treatment of penile carcinoma. Cancer 1977;39:456-66. [PubMed]
- Kosaka T, Ueshige N, Sugaya J, et al. Lymphatic routes of the stomach demonstrated by gastric carcinomas with solitary lymph node metastasis. Surg Today 1999;29:695-700. [PubMed]
- Japanese Society of Biliary Surgery. General Rules for Surgical and Pathological Studies on Cancer of Biliary Tract. 5th ed. Tokyo: Kanehara; 2003.
- Sommerville CA, Limongelli P, Pai M, et al. Survival analysis after pancreatic resection for ampullary and pancreatic head carcinoma: an analysis of clinicopathological factors. J Surg Oncol 2009;100:651-6. [PubMed]
- Sierzega M, Nowak K, Kulig J, et al. Lymph node involvement in ampullary cancer: the importance of the number, ratio, and location of metastatic nodes. J Surg Oncol 2009;100:19-24. [PubMed]
- Hurtuk MG, Hughes C, Shoup M, et al. Does lymph node ratio impact survival in resected periampullary malignancies? Am J Surg 2009;197:348-52. [PubMed]
- Pawlik TM, Gleisner AL, Cameron JL, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery 2007;141:610-8. [PubMed]
- Berger AC, Sigurdson ER, LeVoyer T, et al. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol 2005;23:8706-12. [PubMed]