Down-regulated expressions of PPARγ and its coactivator PGC-1 are related to gastric carcinogenesis and Lauren’s classification in gastric carcinoma
Introduction
Peroxisome proliferator activated receptor-gamma (PPARγ) belongs to a nuclear hormone receptor superfamily that regulates gene expression. PPARγ is composed of four domains. Among them, the DNA binding domain can bind to the peroxisome proliferating response element (PPRE) in the promoters of the target genes specifically. Previous studies showed that treatment with PPARγ agonists such as troglitazone and 15-deoxy-Delta 12,14-prostaglandin J2 had an inhibitory effect on cell proliferation. PPARγ coactivator-1 (PGC-1) family members are coactivators of PPARγ, including PGC-1α, PGC-1β and PGC-1 related coactivator (PRC). PGC-1 coactivator docking to specific transcription factors provides a platform for the recruitment of regulatory protein complexes that exert powerful effects on gene transcription. The N-terminal region of PGC-1 interacts with proteins containing histone acetyltransferase (HAT) activity, including CREB-binding protein/p300 and SRC-1 (1). These proteins acetylate histones and remodel chromatin structure to allow access of the transcriptional machinery to target genes.
Abnormalities in PPARγ have been implicated in tumorigenesis in animal models and human cancers. Down-regulation of PPARγ has been observed in human malignancies such as pulmonary and esophageal cancer, where the low levels of PPARγ expression is thought to correlate with poor prognosis (2,3). In gastric carcinoma (GC), a reduction of PPARγ has been associated with a decrease in E-cadherin and an augmented matrix metalloproteinase-2 (MMP-2) expression (4). PGC-1 plays an important part in regulating the transcriptional activity of PPARγ. Therefore, the abnormalities in PGC-1 expression might serve as an important factor in influencing PPARγ function. Moreover, the crucial role of PGC-1 in controlling mitochondrial biogenesis and scavenging reactive oxygen species (ROS) also implies potential links to tumorigenesis (5). All these findings seem to indicate PGC-1 as a potential tumor suppressor, which is further supported by the detection of decreased PGC-1 expression in human breast, colorectal and prostate cancers (6-8). However, recent studies demonstrated that PGC-1 can activate the production of vascular endothelial growth factor (VEGF) through estrogen-related receptor-α (ERR-α) dependent pathway (9), while VEGF has been established as an important factor in promoting angiogenesis. This link makes the relationship between PGC-1 and cancer more complicated, because PGC-1 might possibly have dual effects on tumorigenesis. In order to address this issue, it is essential to clarify the expression pattern of PGC-1 proteins as an important step into the full understanding of mechanisms behind PGC-1 and human gastric cancers.
Materials and methods
Clinicopathological data and tissue microarray construction
This work was approved by the Institutional Review Board of the First Hospital of China Medical University (No.2010-12). Totally 179 patients with primary GC who underwent curative resection without radiotherapy or chemotherapy at the First Hospital of China Medical University between December 2003 and April 2008 were involved in this study. The specimens consist of 179 cases of GC, 108 cases of matched normal gastric mucosa (obtained at >5 cm apart from the edge of primary tumor focus), 23 chronic atrophic gastritis (CAG), 41 intestinal metaplasia (IM) and 15 dysplasia (Dys). The patients included 125 males and 54 females with the mean age of 61 years. According to Bormann’s classification, gross types of primary tumors were classified as follows: 3 cases of Bormann I, 21 Bormann II, 144 Bormann III, and 11 Bormann IV. According to the World Health Organization’s histological classification of GC, the 179 cases were classified as follows: 2 papillary adenocarcinoma, 13 well and 68 moderately differentiated tubular adenocarcinoma, 73 poorly differentiated adenocarcinoma, 4 undifferentiated carcinoma, 15 mucinous adenocarcinoma and 4 signet ring cell carcinomas (SRC). Samples were fixed in 10% formalin, embedded in paraffin and constructed into tissue microarray. All the samples were evaluated by two experienced pathologists for confirmed diagnosis. Fresh GC tissues and corresponding normal gastric mucosa from 16 patients were analyzed by Western blot for PGC-1 expression. None of the patients had received chemotherapy or radiation therapy preoperatively. Of the 179 cases, 148 patients were evaluated for survival analysis.
Immunohistochemistry (IHC)
Expressions of PPARγ and PGC-1 in GC, precancerous lesions and normal gastric mucosa were detected using IHC method. The PV-9000 kit was purchased from Beijing Zhongshan Golden Bridge Biotechnology Company. Mouse anti-human PPARγ polyclonal antibody was from purchased Santa Cruz (dilution 1:80). Rabbit anti-human PGC-1 polyclonal antibody was from Cayman Chemicals (dilution 1:100). All procedures were implemented according to the manufacturer’s instructions. For negative controls, sections were treated with 0.01 mol/L phosphate-buffered saline (PBS) instead of primary antibodies.
Immunohistochemical staining evaluation
Both the intensity and the extent of staining were assessed. The positive cells of both PPARγ and PGC-1 were defined as that there was clearly brown granules located in nucleus and cytoplasm. Staining intensity was initially recorded on a four-point scale: 0, no staining; 1, light brown; 2, brown; and 3, dark brown. The extent of staining was also initially assessed on a four-point scale: 0, <5% positive cells; 1, 5-25% positive cells; 2, 26-50% positive cells; 3, 51-75% positive cells; and 4, >75% positive cells. According to above assessing criterion, the immunostaining results were classified into: 0-2, negative (–); 3-4, weakly positive (+); 6-8, moderately positive (++); and 9-12, strongly positive (+++). In present study, it was defined as specific positive case that the product of staining intensity and the percentage of positive cells was ≥3.
Western blotting analysis
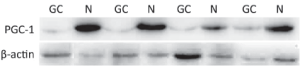
PGC-1 proteins in 16 GC and corresponding normal tissues were detected by Western blotting analysis. Tissue extracts were separated on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel and blotted. Immunodetection was carried out using a PGC-1 antibody (Cayman Chemicals, USA) after overnight incubation at a dilution of 1:500 in Tris-buffered saline (TBS) with 0.5% Tween 20.
Statistical analysis
Categorical data are described using frequencies and percentages. Continuous data are described using means and standard deviations for normally distributed data. Statistical analysis was performed using SPSS 13.0 Package (SPSS Inc., Chicago., IL, USA), and χ2 test or Fisher’s exact test was used to differentiate the rates of different groups. Time-to-event data were estimated by the Kaplan-Meier method and analyzed with the log-rank test. The cumulative overall survival rates were calculated using life table techniques, illustrated by Kaplan-Meier plots. All statistical analysis were two sided, and significance was assigned at P<0.05.
Results
Expression of PPARγ in normal gastric mucosa, CAG, IM, Dys and GC
The immunoreactivity to PPARγ protein was located both in the nucleus and cytoplasms. The positive rate of PPARγ presence in GC (54.75%, 98/179) was significantly lower than that in normal gastric mucosa (70.37%, 76/108) (P=0.009). The positive rate of PPARγ expression in IM (87.8%, 36/41) was significantly higher than that in normal gastric mucosa. The positive rates of PPARγ expression in CAG (86.96%, 20/23) and Dys (86.67%, 13/15) were higher than that in normal mucosa, respectively, but the difference was not significant (P>0.05) (Table 1, Figure 1).
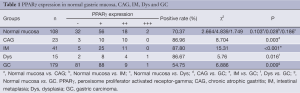
Full table
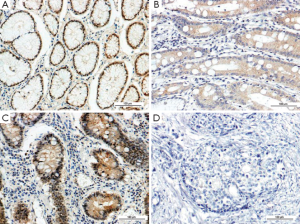
Expression of PGC-1 in normal gastric mucosa, CAG, IM, Dys and GC
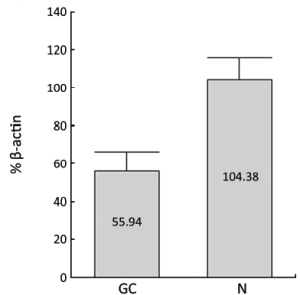
Similar to PPARγ, the immunoreactivity of PGC-1 protein was located in the nucleus and cytoplasms. The positive rate of PGC-1 in GC (49.16%, 88/179) was significantly lower than that in normal gastric mucosa (71.30%, 77/108) (P<0.001). The expressions of PGC-1 in CAG (91.30%, 21/23) and IM (92.68%, 38/41) were also significantly higher than that in normal gastric mucosa, while no significant difference existed between PGC-1 expression in normal gastric mucosa and Dys (60.00%, 9/15), (Table 2, Figure 2). The difference between PGC-1 expression in normal gastric mucosa and GC was further confirmed by Western blotting analysis as shown in Figures 3,4.
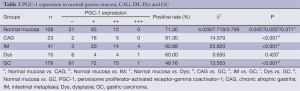
Full table
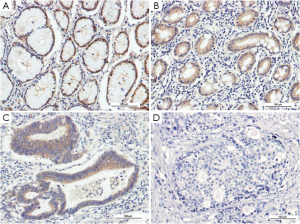
Correlations of PPARγ and PGC-1 expressions with clinicopathological features of GC
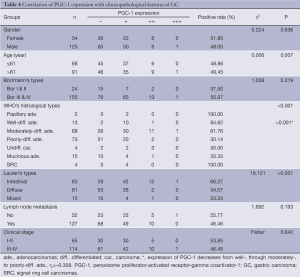
Tables 3,4 showed the correlations of PPARγ and PGC-1 expressions with clinicopathological parameters of GC. Statistical analysis demonstrated that the expression of PPARγ in GC was related to the histological differentiation (P<0.001), Borrmann’s classification (P=0.007) and Lauren’s types (P=0.016), but not related to the patients’ age, gender or lymph node metastasis. There was no relation between PGC-1 expression and gender, age, Bormann’s classification or lymph node metastasis, but PGC-1 expression was significantly higher in intestinal type GC (I-GC) (66.27%) compared with diffuse type one (D-GC) (34.57%) (P<0.001).

Full table
Full table
Correlation between expressions of PPARγ and PGC-1 in GC
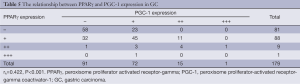
As shown in Table 5, a positive correlation was found between PPARγ and PGC-1 expressions in GC (rk=0.422, P<0.001).
Full table
Impact of PPARγ and PGC-1 expression on survival of patients with GC
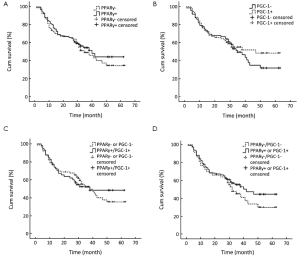
With a total follow-up period of 60 months, 71 of the 148 patients were known to be died. Patients with PPARγ negative tumors tended to have poorer prognosis than patients with PPARγ positive tumors (36.6±3.0 months vs. 38.5±2.7 months). The 5-year survival rates for patients with negative and positive PPARγ expression were 34.4% and 44.1%, respectively, but the difference was not statistically significant (P=0.522, log-rank test) (Figure 5A). The 5-year survival rates of patients with negative and positive PGC-1 expression were 32.0% and 48.2%, respectively, and the survival time of patients with PGC-1 negative expression tended to be shorter than that of patients with PGC-1 positive expression (36.2±2.8 months vs. 39.9±2.9 months). However, the difference was not significant either (P=0.462, log-rank test) (Figure 5B). The prognosis of patients with either PPARγ or PGC-1 negative tumors only differed slightly from that of patients with tumors expressing both PPARγ and PGC-1 (37.5±2.5 months vs. 38.6±3.2 months). The 5-year survival rates were 35.4% and 48.4%, respectively (P=0.875, log-rank test) (Figure 5C). The survival time of patients with both PPARγ and PGC-1 negative tumors was shorter than that of patients with tumors expressing either PPARγ or PGC-1 (34.9±3.3 months vs. 39.4±2.5 months). The 5-year survival rates were 30.0% and 44.7%, respectively, but no statistical significance was shown (P=0.253, log-rank test) (Figure 5D).
Discussion
PPARγ activity can influence carcinogenesis through multiple pathways. One of these effects is relevant to cell cycle control. For example, PPARγ activation can repress the activity of E2F/DP by preventing retinoblastoma (RB) protein from being phosphated thus remain RB active, and the application of PPARγ ligands is able to induce the expression of P21Waf1 and P27Kip1, resulting in cell cycle arrest (10). Another mechanism of PPARγ’s antiproliferative effect involves cellular apoptosis in gastric cancer (11). In another study, PPARγ was shown to bind with the promoter zone of proline oxydase (POX) causing an up-regulation of POX expression, which in turn participate in the mediation of cellular apoptosis by amplifying ROS production (12). These studies provided evidence that, in addition to negatively affecting cell cycle, PPARγ may further inhibit cell proliferation through enhancing the tendency of cellular apoptosis. Meanwhile, PPARγ activation has been further considered as an inhibitor in the process of tumor invasion and metastasis. Conjugated linoleic acid, a selective PPARγ activator, was able to influence the E-cadherin/β-catenin pathway and reduce the invasiveness of breast cancer cells MCF-7 (13). An IHC study showed that a down-regulation of PPARγ in gastric cancer was usually accompanied by a reduction in E-cadherin and an increase in MMP-2 expression, an alteration even more evident in metastatic tissues than that in primary tumors (4).
Decreased PPARγ expression was found in esophageal cancer, lung cancer, follicular thyroid cancer and cervical carcinoma, and correlated with poor prognosis in patients with esophageal cancer and lung cancer (2,3,14,15). Badawi et al. reported that down-regulation of PPARγ mRNA level was characterized as predictors of breast cancer metastases (16). In contrast, some other studies showed that PPARγ expression level was higher in ovarian and pancreatic cancers than that in corresponding normal tissues (17,18). In current study, we found the expression of PPARγ in normal gastric mucosa, CAG, IM and Dys was significantly higher than that in GC. The frequency of samples with positive PPARγ immunohistochemical staining decreased as the differentiation degree turned from well-, through moderately- to poorly-differentiated carcinomas, suggesting a stepwise reduction of PPARγ activity might involved in the histological differentiation of gastric cancer cells and the tumor progression. The down-regulation of PPARγ in gastric cancer tissues shown in our study can be possibly explained by the antiproliferative effects of its activation, which suggests the loss or reduction of PPARγ activity might act as a contributory factor in the development of gastric cancers, or facilitate in their progression. However, the relationship with tumor invasion and metastasis has not been demonstrated, with the positive rates of PPARγ in primary tumors with and without lymph node metastasis very close to each other. But considering the fact that present study is limited to the examination of primary tumors, we speculate a further study including metastatic samples may come up with more objective results. Moreover, the prognosis of patients with PPARγ expression seems to be better, but the association is weak, which may result from the limited number of samples available to the survival analysis.
As PPARγ acts as a potential tumor suppressor, alteration of its coactivator PGC-1 probably influences the process of carcinogenesis through affecting PPARγ activity. Jiang et al. reported abnormal expression of PGC-1 on transcript level in human breast cancer, which is the first report concerning PGC-1 alterations in cancers, suggesting that simultaneous loss of both PPARγ and PGC-1 may be important, for this defect makes the cells unable to respond to either exogenous or endogenous agonists (6). A following study using IHC method showed a down-regulation of both PPARγ and PGC-1 proteins in human breast cancer tissues (19). In our IHC investigation, reduction of PGC-1 protein was also observed in gastric cancers. The expression of PGC-1 in normal gastric mucosa, CAG, IM and Dys was significantly higher than that in gastric cancer, suggesting the reduction of PGC-1 may contribute to malignant transformation of the gastric mucosa. Therefore, we speculate that it is possible for PGC-1 to serve as a tumor suppressing factor in gastric carcinogenesis. Similar to PPARγ, positive PGC-1 staining decreased in a stepwise manner as the differential stage turned from well-, moderately-, to poorly-differentiated cancers. Moreover, in I-GC, the positive rate of PGC-1 was significantly higher than that in diffused and mixed types, indicating that decreased PGC-1 may be associated with the occurrence of diffused and mixed types of GC. However, no significant correlation between PGC-1 expression and lymph node metastasis was observed. In our survival analysis, patients in PGC-1 positive group had a trend to come out with a better prognosis, but no statistical significance was found. The clinical outcome of patients with both PPARγ and PGC-1 positive was not different from other patients, but the outcome of patients with both PPARγ and PGC-1 negative tended to be worse than that of patients with either PPARγ or PGC-1 positive. Though the difference is not statistically significant, this trend is consistent with the argument made in a previous report (6).
Considering the similarity in the alteration of expression pattern of PPARγ and PGC-1, we further examined the relationship between their expressions, and a positive correlation was shown. Treatment with thiazolidinediones (TZDs) and rexinoids in earlier study was shown to induce expression of PGC-1 in white and brown adipocytes. This is due to the presence of PPRE in the distal region of the PGC-1α gene promoter that binds PPARγ/retinoid X receptor heterodimers, thus forming a positive autoregulatory loop of control of PGC-1α gene through coactivation of PPARγ responsiveness to TZDs by PGC-1α itself. A similar regulation may also exist in gastric mucosa, and if it does, the correlation between PGC-1 and PPARγ can be reasonably explained, since activation of PPARγ itself can act as stimulator of PGC-1 expression. The results of these studies support the idea that abnormal PGC-1 expression participates in tumor development, and the mechanisms are probably relevant to PPARγ.
However, the roles of PGC-1 in cancers may extend to mechanisms independent of PPARγ. One of these mechanisms possibly involves ROS production in mitochondria. The mitochondrial electron transport chain is a major site of ROS production. Due to the close proximity to the electron transport chain, mitochondrial DNA (mtDNA) is very susceptible to the damage from endogenous ROS, causing mtDNA mutations. Mutations in mtDNA could in turn cause further increases of ROS production due to the loss of certain electron transport chain components, thus leading to additional mutations and oxidative stress. A moderate increase of ROS has been found to stimulate cellular proliferation, while augmented ROS production can even further facilitate cancer metastasis (20). As a promoting factor of energy production, PGC-1 can stimulate the expression of superoxide dismutase (SOD) and glutathione peroxidase, as well as enzymes responsible for glutathione biosynthesis while promoting mitochondrial-based respiration, thereby enabling cells to maintain normal redox status in response to changing oxidative capacity. Moreover, PGC-1α and β also stimulate the expression of uncoupling protein-2 (UCP2) and UCP3. These proteins can dissipate the proton gradient and lower mitochondrial membrane potential, which is thought to remarkably reduce ROS production by mitochondria (5).
In addition, a deficiency of mtDNA has also been found in a number of solid tumors, including gastric cancers, which might account for the decrease of respiratory chain proteins, and have relations with the clinical features (21). Nonetheless, the specific mechanisms behind the decrease of mtDNA copy number in cancers have hardly been revealed yet. However, PGC-1 family members are probably implicated in this connection, because besides of nuclear receptors (NRs) such as PPARγ, PGC-1α also coactivates non-NR transcription factors, such as nuclear respiratory factor-1 (NRF-1) and NRF-2. NRFs regulate expression of mitochondrial transcription factor A (TFAM), a nuclear-encoded transcription factor essential for replication, maintenance, and transcription of mitochondrial DNA. NRF-1 and NRF-2 also control the expression of nuclear genes encoding respiratory chain subunits and other proteins required for mitochondrial function (22). These facts suggest PGC-1α play a vital part in mitochondrial biogenesis and cell energy metabolism. Thus, it is quite possible that the down-regulation of PGC-1 found in present study contributes to the reduction of mtDNA copies in gastric cancers.
All these findings seemed to support PGC-1 as a tumor suppressing factor. However, in a recent study, Arany et al. reported that PGC-1α up-regulates the release of VEGF through ERR-α, an orphan nuclear receptor and well-known partner of PGC-1α. This molecular link ensures that consumption of oxygen by oxidative metabolism remains in close balance with supply of oxygen through angiogenesis to meet the metabolic needs of tissues, a mechanism also serving in cancer tissue under rapid growth (9). Since the formation of new vessels is a critical step in cancer invasion and progression, the pro-angiogenesis property of PGC-1α probably associates its expression with a greater metastatic tendency and a poorer prognosis, making the issue of how PGC-1 expression affects cancer more complicated.
In conclusion, our study demonstrates a reduction of both PPARγ and PGC-1 in GC comparing with normal gastric mucosa and IM tissues, and these alterations are associated with certain clinicopathological parameters as shown above. These results suggest decreased PPARγ and PGC-1 probably play important roles in gastric cancinogenesis, and the correlation between their expressions supports the assumption that their activities may be closely related in gastric cancers. However, PPARγ and PGC-1 have not been shown to influence lymph node metastasis and clinical prognosis in this study, which might result from the limit of available samples, or from the effect of PGC-1 on VEGF. The weak effect of PGC-1 on prognosis showed in our study is probably a reflection of this dual effect. To clarify the relations between PGC-1 and VEGF might require further investigation. The complex interactions of PGC-1 with PPARγ, ROS, mtDNA and VEGF might mean it plays an important role in integrating extensive cell activities. While the understanding of complex networks within cells is an inevitable step towards the full comprehension of underlying mechanisms behind cancer development and progression, the study of PGC-1 is probably an opportunity in deepening our knowledge on the relationship between cancinogensis and multiple cellular activities, especially those related to cell energy metabolism.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81071650; 30973503) and the Supporting Project for Climbing Scholars in Liaoning Provincial Universities, China (2009-2012).
Disclosure: The authors declare no conflict of interest.
References
- Puigserver P, Adelmant G, Wu Z, et al. Activation of PPARγ coactivator-1 through transcription factor docking. Science 1999;286:1368-71. [PubMed]
- Sasaki H, Tanahashi M, Yukiue H, et al. Decreased perioxisome proliferator -activated receptor gamma gene expression was correlated with poor prognosis in patients with lung cancer. Lung Cancer 2002;36:71-6. [PubMed]
- Terashita Y, Sasaki H, Haruki N, et al. Decreased peroxisome proliferator -activated receptor gamma gene expression is correlated with poor prognosis in patients with esophageal cancer. Jpn J Clin Oncol 2002;32:238-43. [PubMed]
- He Q, Chen J, Lin HL, et al. Expression of peroxisome proliferator-activated receptor γ, E-cadherin and matrix metalloproteinases-2 in gastric carcinoma and lymph node metastases. Chin Med J 2007;120:1498-504. [PubMed]
- Miwa S, Brand MD. Mitochondrial matrix reactive oxygen species production is very sensitive to mild uncoupling. Biochem Soc Trans 2003;31:1300-1. [PubMed]
- Jiang Y, Zou L, Zhang C, et al. PPARgamma and Wnt/beta-Catenin pathway in human breast cancer: expression pattern, molecular interaction and clinical/prognostic correlations. J Cancer Res Clin Oncol 2009;135:1551-9. [PubMed]
- Feilchenfeldt J, Bründler MA, Soravia C, et al. Peroxisome proliferator-activated receptors (PPARs) and associated transcription factors in colon cancer: reduced expression of PPARγ-coactivator 1 (PGC-1). Cancer Lett 2004;203:25-33. [PubMed]
- Jiang WG, Davies G, Kynaston H, et al. Does the PGC-1/PPARγ pathway play a role in Com-1/p8 mediated cell growth inhibition in prostate cancer? Int J Mol Med 2006;18:1169-75. [PubMed]
- Arany Z, Foo SY, Ma Y, et al. HIF-independent regulation of VEGF and angiogenesis by the transcription coactivator PGC-1α. Nature 2008;451:1008-12. [PubMed]
- Yu HN, Lee YR, Noh EM, et al. Induction of G1 phase arrest and apoptosis in MDA-MB-231 breast cancer cells by troglitazone, a synthetic peroxisome proliferator-activated receptor gamma (PPARgamma) ligand. Cell Biol Int 2008;32:906-12. [PubMed]
- Ramachandran L, Manu KA, Shanmugam MK, et al. Isorhamnetin inhibits proliferation and invasion and induces apoptosis through the modulation of peroxisome proliferator-activated receptor γ activation pathway in gastric cancer. J Biol Chem 2012;287:38028-40. [PubMed]
- Wang J, Lv X, Shi J, et al. Troglitazone induced apoptosis via PPARγ activated POX-induced ROS formation in HT29 cells. Biomed Environ Sci 2011;24:391-9. [PubMed]
- Bocca C, Bozzo F, Francica S, et al. Involvement of PPAR γ and E-cadherin/beta-catenin pathway in the anti-proliferative effect of conjugated linoleic acid in MCF-7 cells. Int J Cancer 2007;121:248-56. [PubMed]
- Jung TI, Baek WK, Suh SI, et al. Down-regulation of peroxisome proliferator -activated receptor gamma in human cervical carcinoma. Gynecol Oncol 2005;97:365-73. [PubMed]
- Kato Y, Ying H, Zhao L, et al. PPARgamma insufficiency promotes follicular thyroid carcinogenesis via activation of the nuclear factor-kappaB signaling pathway. Oncogene 2006;25:2736-47. [PubMed]
- Badawi AF, Badr MZ. Expression of cyclooxygenase-2 and peroxisome proliferator-activated receptor-gamma and levels of prostaglandin E2 and 15-deoxy-delta12,14-prostaglandin J2 in human breast cancer and metastasis. Int J Cancer 2003;103:84-90. [PubMed]
- Giaginis C, Katsamangou E, Tsourouflis G, et al. Peroxisome proliferator -activated receptor-gamma and retinoid X receptor-alpha expression in pancreatic ductal adenocarcinoma: association with clinicopathological parameters, tumor proliferative capacity, and patients' survival. Med Sci Monit 2009;15:BR148-56. [PubMed]
- Davidson B, Hadar R, Stavnes HT, et al. Expression of the peroxisome proliferator-activated receptors-alpha, -beta, and -gamma in ovarian carcinoma effusions is associated with poor chemoresponse and shorter survival. Hum Pathol 2009;40:705-13. [PubMed]
- Watkins G, Douglas-Jones A, Mansel RE, et al. The localisation and reduction of nuclear staining of PPARgamma and PGC-1 in human breast cancer. Oncol Rep 2004;12:483-8. [PubMed]
- Ishikawa K, Takenaga K, Akimoto M, et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 2008;320:661-4. [PubMed]
- Wallace DC. Mitochondria and cancer. Nat Rev Cancer 2012;12:685-98. [PubMed]
- Gleyzer N, Vercauteren K, Scarpulla RC. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol 2005;25:1354-66. [PubMed]


