Clinicopathological and prognostic role of MMP-9 in esophageal squamous cell carcinoma: a meta-analysis
Introduction
Esophageal cancer is one of the most aggressive malignant tumors. The incidence of esophageal adenocarcinoma rose rapidly in the western country in the past two to three decades, esophageal squamous cell carcinoma (ESCC), however, was still much more common in Asian countries, especially in China and Japan (1-3). Despite recent advances in diagnosis and treatment, the overall prognosis for ESCC is still worse than other digestive tract cancers, with 5-year survival of 5-45% (4,5). The treatment failure of ESCC can be explained by the extensive local invasion and regional lymph node metastasis, which make it difficult to completely resect tumors (6).
The migration of tumor cells is associated with the degradation of the extracellular matrix, which could be induced by various proteolytic enzymes, including matrix metalloproteinases (MMPs) (7). There are at least 28 members in the MMP family, and studies had shown that overexpression of MMPs promoted tumor cell detachments and metastasis which resulted in malignant transformation and poor clinical outcome (8-10).
As a member of MMP family, MMP-9 works directly to degrade the extracellular matrix, and was reported to participate in the development of gastric cancer (11), breast cancer (12), colorectal cancer (13), and non-small cell lung cancer (14), and resulted in the poor prognosis of them. Many studies also researched the correlation between MMP-9 expression and ESCC, however, the numbers of patients they included were small, and the conclusions they drew were inconsistent. For example, Gu et al. (7) and Tanioka et al. (15) reported that MMP-9 was a negative, independent prognostic factor in ESCC and correlated with tumor cell differentiation, vessel permeation, and lymph node metastasis; Lu et al. (3) and Zhu et al. (16), however, reported that there was no correlation between MMP-9 expression and the clinicopathological characteristics of ESCC, and MMP-9 was not a prognostic factor. This made researchers and clinicians confused, and a meta-analysis was needed to explore the issue clearly. The present meta-analysis evaluated all the studies assessing the correlation between MMP-9 expression and ESCC to explore whether MMP-9 participated in the development of ESCC and whether MMP-9 could be a prognosis factor in ESCC.
Materials and methods
Inclusion and exclusion criteria
Studies were included if they met the following criteria: (I) evaluated the correlation between MMP-9 expression and the clinicopathological parameters or overall survival (OS) of ESCC; (II) assessed MMP-9 expression in the primary ESCC tissues; and (III) were published as full texts. The exclusion criteria were as follow: (I) letters, reviews, case reports, conference abstracts, or editorials; (II) articles had no sufficient data to calculate odds ratio (OR) or hazard ratio (HR); and (III) studies had duplication data.
Search strategy
We conducted the meta-analysis following preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (17). PubMed, EMBASE, Web of Science and Chinese Biomedical Literature Database were searched from their start year up to Mar. 2013 using the terms of “MMP-9”, “ESCC”. The Mesh terms and their variations were used and no restriction on language was applied. In addition, Google Scholar and other databases were also searched.
Study screening and data extraction
Two reviewers screened each study independently to determine whether it met the inclusion criteria, and resolved disagreements by consensus. For each included study, Zeng R and Duan L independently extracted the following data using a standard form: first author, year of publication, study location, number of patients, test method, cut-off value for positive MMP-9 expression, treatments, clinicopathological parameters (differentiation, lymph node metastasis, TNM stage, the depth of invasion, vascular invasion) and patient survival results.
Methodological assessment
The Newcastle-Ottawa quality assessment scale (18) was used to assess the methodological quality of included studies. The scale assigned 0-9 stars to each study based on three categories (selection, comparability and outcome).
Statistical analysis
For clinicopathological parameters, ORs with 95% confidence intervals (CIs) were calculated. For the outcome of OS, HR with 95% CI was calculated. For studies had not reported HRs directly, we estimated them from available data using methods reported by Tierney et al. (19) and Zhang et al. (11). Heterogeneity of the studies was evaluated using the χ2 test and the I2 statistic. P<0.1 or I2>50% was considered to be significant for heterogeneity, and a random effects model was used to pool the data, otherwise, a fixed effects model was used. All statistical analyses were conducted using Review Manager 5.2.
For the outcome of OS, subgroup analysis was conducted by different cut-off values for positive MMP-9 expression. We also conducted sensitive analysis only including HRs calculated by multivariate analysis to evaluate whether MMP-9 was an independent prognostic factor in ESCC.
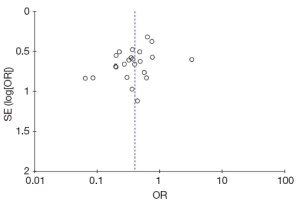
A funnel plot recommended by the Cochrane Handbook (20) was made to explore whether publication bias existed in the meta-analysis or not.
Results
Study selection and characteristics
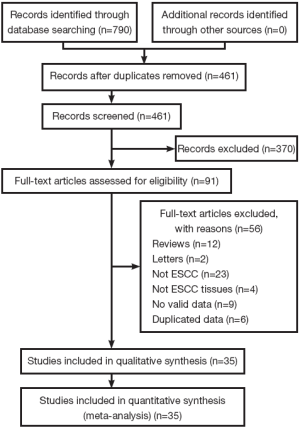
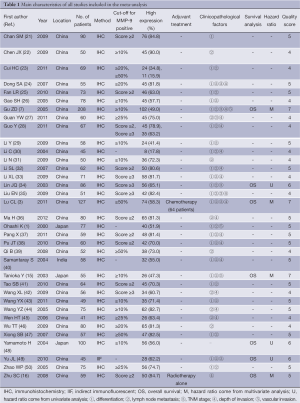
The literature search was performed in March 2013 and 790 potential relevant papers were identified, Figure 1 details the selection process. Finally, 15 studies (1,3,7,15,16,21-30) and 20 other more (31-50) are include in the meta-analysis, with a total of 2,442 patients. These studies investigate the correlation between MMP-9 expression and ESCC were included in the meta-analysis. The studies were conducted between 2000 and 2012, and the numbers of ESCC patients they included ranged from 41 to 208. One study investigated ESCC patients from India (40), three from Japan (1,15,48), and the others from China. Almost all the studies used immunohistochemical analysis to examine the expression of MMP-9 in ESCC tissues except one study (49), which used indirect immunofluorescent analysis. Only one study had not reported the correlation between MMP-9 expression and ESCC clinicopathological parameters (48). Seven studies reported OS and had available data to calculate HRs (3,7,15,16,34,48,49). Table 1 shows the main characteristics of all the included studies.
Full table
The Newcastle-Ottawa quality assessment scale was used to assess the methodological quality of included studies. The numbers of stars achieved by them ranged from 4 to 7: 13 studies achieved 4 stars, 16 studies achieved 5, 3 studies achieved 6, and 3 studies achieved 7.
Meta-analysis results
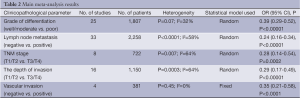
There were 34 studies reported the correlation between MMP-9 expression and clinicopathological characteristics of ESCC. As shown in Table 2, the results of meta-analysis showed that overexpression of MMP-9 was associated with grade of differentiation (well/moderate vs. poor: OR: 0.39, 95% CI: 0.29-0.52; P<0.00001), lymph node metastasis (negative vs. positive: OR: 0.24, 95% CI: 0.16-0.34; P<0.00001), TNM stage (T1/T2 vs. T3/T4: OR: 0.28, 95% CI: 0.14-0.54; P=0.0002), the depth of invasion (T1/T2 vs. T3/T4: OR: 0.29, 95% CI: 0.17-0.49; P<0.00001), and vascular invasion (negative vs. positive: OR: 0.35, 95% CI: 0.21-0.58; P<0.0001).
Full table
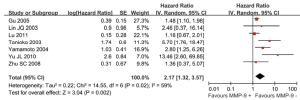
Seven studies reported the outcome of OS and had available data to calculate HRs. However, significant heterogeneity existed among them (I2=59%, P=0.02), so, a random-effects model was used to pool the data, and the results showed that overexpression of MMP-9 was associated with significant increase of mortality risk (HR: 2.17, 95% CI: 1.32-3.57; P=0.002), as shown in Figure 2. Subgroup analysis showed that more than 10% of carcinoma cell staining (i.e., chose ≥10% as cut-off value for MMP-9 positive) was associated with significant increase of mortality risk (HR: 2.44, 95% CI: 1.16-5.15; P=0.02), as shown in Table 3. Sensitive analysis only including HRs calculated by multivariate analysis also showed that MMP-9 overexpression was correlated with poor survival (HR: 1.49, 95% CI: 1.16-1.91; P=0.002), as shown in Table 3.

Full table
Figure 3 shows the funnel plot for the outcome of the correlation between MMP-9 expression and the grade of ESCC differentiation, and it showed no asymmetry exhibiting, demonstrating that there was probably no publication bias.
Discussion
The present study is the first meta-analysis exploring the correlation between MMP-9 expression and the clinicopathological characteristics and prognosis of ESCC. We analyzed the data of 2,442 ESCC patients from 35 individual studies, and showed that overexpression of MMP-9 was significantly associated with the depth of invasion, lymph node metastasis, distant metastasis, vascular invasion and grade of differentiation. The most important finding of the present study was that overexpression of MMP-9 was associated with poor OS of ESCC patients.
MMPs are multi-domain, zinc-dependent endopeptidases, and all of them share homologous amino acid (AA) sequences (9,48). The structure of most MMPs (except MMP-7, -23 and -26) includes a propeptide containing about 80 AA, a catalytic domain with about 170 AA, followed by a linker peptide called the ‘hinge region’, of variable length, and a hemopexin domain of about 200 AA (10). There are six categories in the MMP family: collagenases, gelatinases, stromelysins, matrilysins, membrane-type MMPs, and other MMPs (10).
As proteolytic enzymes, MMPs play important roles in the degradation of the extracellular matrix, leading to the proteolysis of microvessel basement membranes and invasion of endothelium, which contribute highly to the first step of tumor development and metastasis (8,9,48).
MMP-9, categorized in the subgroup of gelatinases, works directly to degrade the extracellular matrix (10). Many systematic reviews reported MMP-9 participated in the development of cancers, and resulted in poor prognosis. Zhang et al. (11) conducted a meta-analysis including 11 studies (covering 1,700 patients with gastric cancer), and reported that positive MMP-9 expression had an adverse impact on OS in gastric cancer patients (HR: 1.25, 95% CI: 1.11-1.40). A meta-analysis conducted by Song et al. (12) analyzed 15 studies (including 2,344 breast cancer patients), and the results suggested that MMP-9 overexpression had an unfavorable impact on both OS (HR: 1.70, 95% CI: 1.41-2.04) and relapse free survival (RFS) (HR: 1.54, 95% CI: 1.17-2.01) in breast cancer patients. The results of the meta-analysis conducted by Li et al. (13) (including 1,379 colorectal cancer patients) also found that MMP-9 overexpression was associated with poorer OS (HR: 1.78, 95% CI: 1.31-2.41) as well as RFS (HR: 1.92, 95% CI: 1.46-2.53) in patients with colorectal cancer. Seventeen studies covering 2,029 non-small cell lung cancer cases were analyzed by the study of Peng et al. (14), and the combined HR of 1.84 (95% CI: 1.62-2.09) suggested that MMP-9 overexpression was associated with a poor prognosis in patients with NSCLC.
The correlation between MMP-9 expression and ESCC was also explored by many studies, however, the patients they included were too few to draw a firm conclusion. Moreover, the studies had different cut-off values for positive MMP-9 expression and different adjuvant treatments, which made them had inconsistent conclusions (3,7,15,16). The present study conducted a comprehensive search for related studies, and finally included 35 studies (including 2,442 patients) to investigate whether MMP-9 was correlated with clinicopathological characteristics of ESCC and whether MMP-9 could be a prognostic factor in ESCC. The results of meta-analysis showed that overexpression of MMP-9 was associated with poor cell differentiation, poor TNM stage, lymph node metastasis, vascular invasion as well as poor prognosis of ESCC, which suggested that MMP-9 participated in the development of ESCC and could be a prognostic factor in ESCC. We also conducted sensitive analysis only including HRs calculated by multivariate analysis (Cox proportional hazards model), and found that MMP-9 was an independent prognostic factor in ESCC (HR: 1.49, 95% CI: 1.16-1.91; P=0.002). Given the included studies were limited, we only conducted subgroup analysis on studies choosing 10% staining as cut-off value, but we failed to conducted subgroup analysis by different adjuvant treatments. Future studies should focus on what cut-off value is optimal, and whether different treatments affect the prognostic power of MMP-9.
Our study had several limitations. Firstly, only seven studies (including 680 ESCC patients) had available data to calculate HRs, which made it difficult to draw a firm conclusion on the prognostic value of MMP-9 for ESCC. Secondly, given all the included studies investigated ESCC patients from Asia, especially from China and Japan, the results may just represent the correlation between MMP-9 expression and ESCC patients from Asia. Thirdly, most included studies had poor methodological quality, which just achieved four to five stars assessed by the Newcastle-Ottawa quality assessment scale, and most of them did not had follow-up period long enough.
In conclusion, the meta-analysis suggested that MMP-9 participated in the development of ESCC, and may be a potential independent prognostic factor in ESCC patients in Asia. High-quality studies assessing the prognostic significance of MMP-9 for ESCC patients are still needed.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ohashi K, Nemoto T, Nakamura K, et al. Increased expression of matrix metalloproteinase 7 and 9 and membrane type 1-matrix metalloproteinase in esophageal squamous cell carcinomas. Cancer 2000;88:2201-9. [PubMed]
- Li T, Lu ZM, Chen KN, et al. Human papillomavirus type 16 is an important infectious factor in the high incidence of esophageal cancer in Anyang area of China. Carcinogenesis 2001;22:929-34. [PubMed]
- Lu CL, Ji Y, Ge D, et al. The expression of CXCR4 and its relationship with matrix metalloproteinase-9/vascular endothelial growth factor in esophageal squamous cell cancer. Dis Esophagus 2011;24:283-90. [PubMed]
- Roder JD, Busch R, Stein HJ, et al. Ratio of invaded to removed lymph nodes as a predictor of survival in squamous cell carcinoma of the oesophagus. Br J Surg 1994;81:410-3. [PubMed]
- Sato F, Shimada Y, Watanabe G, et al. Expression of vascular endothelial growth factor, matrix metalloproteinase-9 and E-cadherin in the process of lymph node metastasis in oesophageal cancer. Br J Cancer 1999;80:1366-72. [PubMed]
- Wang LS, Chow KC, Chi KH, et al. Prognosis of esophageal squamous cell carcinoma: Analysis of clinicopathological and biological factors. Am J Gastroenterol 1999;94:1933-40. [PubMed]
- Gu ZD, Li JY, Li M, et al. Matrix metalloproteinases expression correlates with survival in patients with esophageal squamous cell carcinoma. Am J Gastroenterol 2005;100:1835-43. [PubMed]
- Zeng ZS, Cohen AM, Guillem JG. Loss of basement membrane type IV collagen is associated with increased expression of metalloproteinases 2 and 9 (MMP-2 and MMP-9) during human colorectal tumorigenesis. Carcinogenesis 1999;20:749-55. [PubMed]
- Ii M, Yamamoto H, Adachi Y, et al. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med 2006;231:20-7. [PubMed]
- Groblewska M, Siewko M, Mroczko B, et al. The role of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) in the development of esophageal cancer. Folia Histochem Cytobiol 2012;50:12-9. [PubMed]
- Zhang QW, Liu L, Chen R, et al. Matrix metalloproteinase-9 as a prognostic factor in gastric cancer: a meta-analysis. Asian Pac J Cancer Prev 2012;13:2903-8. [PubMed]
- Song J, Su H, Zhou YY, et al. Prognostic value of matrix metalloproteinase 9 expression in breast cancer patients: a meta-analysis. Asian Pac J Cancer Prev 2013;14:1615-21. [PubMed]
- Li CY, Yuan P, Lin SS, et al. Matrix metalloproteinase 9 expression and prognosis in colorectal cancer: a meta-analysis. Tumour Biol 2013;34:735-41. [PubMed]
- Peng WJ, Zhang JQ, Wang BX, et al. Prognostic value of matrix metalloproteinase 9 expression in patients with non-small cell lung cancer. Clin Chim Acta 2012;413:1121-6. [PubMed]
- Tanioka Y, Yoshida T, Yagawa T, et al. Matrix metalloproteinase-7 and matrix metalloproteinase-9 are associated with unfavourable prognosis in superficial oesophageal cancer. Br J Cancer 2003;89:2116-21. [PubMed]
- Zhu SC, Wang YF, Su JW, et al. MMP-9, uPA and uPAR proteins expression and its prognostic significance in esophageal squamous cell carcinoma treated by radiotherapy. Zhong Hua Fang She Zhong Liu Xue Za Zhi 2008;17:263-7.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [PubMed]
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [PubMed]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available online: www.cochrane-handbook.org
- Chan SM, Ou XL, Sun WH, et al. Expression of cyclooxygenase-2 and matrix metalloproteinase-9 in human esophageal squamous cell carcinoma and its correlation with angiogenesis. Shi Jie Hua Ren Xiao Hua Za Zhi 2009;17:2259-65.
- Chen JX, Cai KJ, Chen YX. Relationship between metastasis of esophageal squamous cell carcinoma and expression of CD147, MMP9 and S100A4. Zhong Liu Fang Zhi Yan Jiu 2009;36:596-9.
- Cui HC, Song PA, Yao Y, et al. Expression of MMP-9 and E-cadherin in esophageal carcinoma and its significance. Zhong Guo Lin Chuang Yan Jiu (in Chinese) 2011;24:460,461,484,553.
- Dong SA, Zhao JL, Lin LS. Expression of MMP-2, MMP-9 and their tissue inhibitor TIMP-1 in esophageal carcinoma and their clinical significance. Qing Dao Da Xue Yi Xue Yuan Xue Bao 2007;43:105-7.
- Fan LR, Xu ZQ, Chen FR, et al. Expression and significance of CD44v6 and MMP-9 in human esophagus squamous cell carcinoma. Zhong Liu Ji Chu Yu Lin Chuang 2010;23:195-8.
- Gao SH, Zhao JP, Pan TC, et al. Expression of MMP-9 and TIMP-1 and its correlation with lymph node metastasis in esophageal squamous carcinoma. Zhong Guo Yi Shi Za Zhi 2005;7:1016-8.
- Guan YW. Expression and significance of VEGF and MMP-9 in human esophageal squamous cell carcinoma. Shan Dong Yi Yao 2011;51:86-7.
- Guo Y, Wang LD, Hao XQ, et al. Overexpression of MMP-2 and MMP-9 in esophageal squamous cell carcinoma. Zhong Guo Lao Nian Xue Za Zhi 2011;31:389-91.
- Li Y, Ma J, Guo Q, et al. Overexpression of MMP-2 and MMP-9 in esophageal squamous cell carcinoma. Dis Esophagus 2009;22:664-7. [PubMed]
- Li C, Wu MY, Wu XY. Expressions of MMP-2, MMP-9 and its tissue inhibitor TIMP-1, TIMP-2 in esophageal carcinoma and its clinical significance. Shi Yong Ai Zheng Za Zhi 2004;19:139-41.
- Li N, Li SS, Zhang HY, et al. Function and correlation of KISS-1 and MMP-9 in human esophagus squamous cell carcinoma. Zhong Hua Xiao Hua Za Zhi 2009;29:345-6.
- Li SL, Zhao QM, Liu ZW, et al. Correlation between the protein expression of reversion inducing cysteine rich protein with Kazal motifs and matrix metalloproteinase-9 in esophageal squamous cell carcinoma and its clinical pathological significance. Shi Jie Hua Ren Xiao Hua Za Zhi 2007;15:1082-6.
- Li XL, Liu EN, Chen KS. Expression of MMP-9 and E-Cad protein in esophageal squamous cell carcinoma. Yi Yao Lun Tan Za Zhi 2009;30:23-5.
- Lin JQ, Zhu SZ, Lin RB. Studies on the clinical significance of matrix metalloproteinase-9 and tissue inhibitors of metalloproteinase-1 expression in esophageal squamous carcinoma. Zhong Guo Zhong Liu Lin Chuang 2003;30:708-11.
- Liu EN, Wang XL, Chen KS. Expression of VEGF and MMP-9 protein in esophageal squamous cell carcinoma. Yi Yao Lun Tan Za Zhi 2009;30:3-5.
- Ma H, Li L, Haliha S, et al. Expression of TLR4 and TLR9/NF-κB in esophageal squamous cell carcinoma and their relationship with MMP-9. Zhong Liu Fang Zhi Yan Jiu 2012;39:1017-9.
- Pang X, Li SL, Zhao ZH, et al. Expression and clinical significance of FAK, Paxillin and MMP-9 protein in esophageal squamous cell carcinoma tissue. Chong Qing Yi Xue 2011;40:1367-9.
- Pu JT, Dai TY, Yang Z. The expression and clinical significance of MMP-9, VEGF in the esophageal squamous cell carcinomas. Zhong Guo Ji Ceng Yi Yao 2010;17:2200-1.
- Qi B, Shi QZ, Liu SG, et al. Expression of matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1 and CD147 in esophageal squamous cell cancer. Yi Xue Xin Xi 2008;21:886-8.
- Samantaray S, Sharma R, Chattopadhyaya TK, et al. Increased expression of MMP-2 and MMP-9 in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol 2004;130:37-44. [PubMed]
- Tao SB, Zhang L, Zhang YH, et al. Expression and clinical pathological significance of MTA1, MMP-9 and MVD in human esophageal squamous cell carcinoma. He Nan Da Xue Xue Bao 2010;29:29-33.
- Wang XL, Li XL, Chen KS. Relationship between protein expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in esophageal squamous cell carcinoma and tumor invasion and metastasis. Yi Yao Lun Tan Za Zhi (in Chinese) 2009;30:1-3,6.
- Wang YX, Li DM, Li HM, et al. Expression and significance of NF-κB p65 and MMP-9 in esophageal squamous cell carcinoma. Guang Dong Yi Xue 2011;32:2401-3.
- Wang YZ, Wu JN, Sun RQ, et al. Expression of MMP-9 and MMP-9 mRNA in esophageal carcinoma and its correlation with antioncogene p53. Jiang Su Da Xue Xue Bao 2005;15:113-6.
- Wen HT, Zhang L, Li JC. Express of MMP-9 and TIMP-1 in human esophageal squamous cell carcinoma. Wei Chang Bing Xue He Gan Bing Xue Za Zhi 2006;15:183-5.
- Wu TT, Yang QX, Zhu SL. Expression of MMP-9 and TIMP-1 in human esophageal squamous cell carcinoma. Dang Dai Yi Xue 2009;15:27-8.
- Xiong SB, Xu Y, Jiang B, et al. Expression of matrix metalloproteinase-9 and CD-147 in human esophageal squamous cell carcinoma. Xian Dai Sheng Wu Yi Xue Jin Zhan (in Chinese) 2007;7:42,43,46.
- Yamamoto H, Vinitketkumnuen A, Adachi Y, et al. Association of matrilysin-2 (MMP-26) expression with tumor progression and activation of MMP-9 in esophageal squamous cell carcinoma. Carcinogenesis 2004;25:2353-60. [PubMed]
- Yu JL, Wen JH. Expression of integrin-linked kinase and matrix metalloproteinase-9 and their significance in esophageal squamous carcinoma tissues. Chong Qing Yi Ke Da Xue Xue Bao 2010;35:1874-7.
- Zhao WP, Xu JY, Zhu J. Expression and significance of matrix metalloproteinase-9 in esophageal squamous cell carcinoma. Shi Yong Zhong Liu Xue Za Zhi 2004;18:439-41.