Retrospective analysis of patients with rare-site and metastatic giant cell tumor
Introduction
A giant cell tumor is a benign bone tumor, accounting for 4% to 9.5% of all bone tumors and 20% of all benign bone tumors. A giant cell tumor occurs mainly in the proximal tibia, humerus, distal radius bone and the pelvic bone (1). It is rarely observed in such sites as the ribs and the temporal bone, though the local recurrence rate is up to 5% to 25% (2). The effect of radiotherapy and chemotherapy after surgery has remained unknown. Most postoperative metastases occur in the lungs, with a reported incidence between 1% and 9% (3,4), followed by the mediastinum and aortic lymph node (5), skin (6) and the breasts (7). The mortality associated with lung metastases of giant cell tumors is 23% (4). In this study, we retrospectively analyzed the long-term outcomes of patients with rare giant cell tumors at the head and neck following surgery, and summarized the treatment options and prognoses for those complicated with lung metastases. One case with locally invasive giant cell tumor of the rib who experienced successful surgical resection following neoadjuvant therapy was reported.
Materials and methods
We retrospectively collected the data of 80 patients diagnosed with giant cell tumors in the pathological department of our hospital from January 2002 to June 2012. Among them, 46 patients were confirmed upon pathology consultation; 23 patients were referred to other hospitals because there was no department of bone surgery in our hospital; 11 patients received treatment and follow-up in our hospital, including six with the lesions at the head and neck, and five with bilateral lung metastases received treatment in the departments of head and neck surgery, chemotherapy and thoracic surgery due to bilateral lung metastases, respectively. The complete treatment and follow-up data were available. In addition, in November 2012, we administered neoadjuvant chemotherapy to one patient with giant cell tumor of the left rib complicated with bloody pleural effusions, followed by partial resection and chest wall reconstruction after the effusions disappeared. The treatment and outcomes of giant cell tumors are analyzed in this study based on the above data and literature.
Results
Patients’ characteristics
Eighty patients were diagnosed in the pathology department in our hospital (Table 1), including 46 men and 34 women, with a mean age of 28 years (0-60 years). There were lesions of long bones in 53 patients (66%), lesions of head and neck bones in 12 patients (15%), pelvic bone area in six patients (7.5%), vertebral bones in two patients (2.5%), ribs in three patients (4%), and unknown primary site on record in four patients (5%).
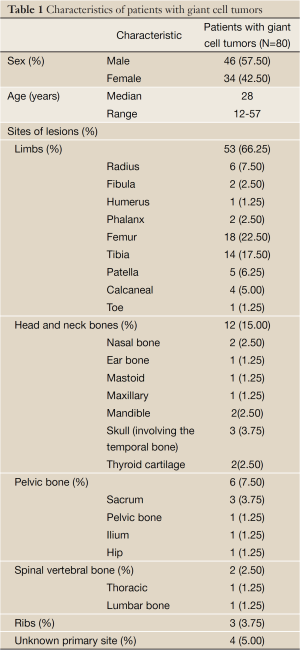
Full Table
From Jun 2002 to June 2012, eleven patients were treated in our hospital, including six patients admitted into the department of head and neck surgery for surgery (7.5%, Table 2). An average follow-up of 42.6 months (until December 19, 2012) was conducted (from 14 to 90 months), and all patients were alive without recurrence. Five patients (6%) admitted due to lung metastases (Table 3) received surgery and chemotherapy in the departments of thoracic surgery and chemotherapy, respectively. One of them received resection of chest wall and lung metastases, which included not only the huge chest wall metastatic lesion but also the lung metastases. One of them received thoracoscopic palliative resection, in which one of the largest lung metastases was resected. Both patients were current alive during follow-up. The three other patients received systemic chemotherapy (Table 4). One of them achieved PR after four cycles of CTX + ADM + DDP, PD after two sequential cycles of IFO+VP-16, and SD after two cycles of BLM + CBP + ACD-t. During the follow-up study four months after the completion of all chemotherapy until two years later, the lung lesions of both sides continued to shrink, showing a tendency of spontaneous regression. One patient achieved PD after two cycles of CTX + ADM + DDP, and remained SD after four sequential cycles of MAID. Since degree 4 neutropenia with pneumonia was present after two cycles of MAID, the patient switched to biological treatment (eight cycles of CIK cell reinfusion for nine months) after 4 cycles of MAID. The last patient achieved SD after three cycles of MTX/CF, and remained SD after two cycles of E-ADM + IFO + endostar. This patient was lost to follow-up after six months. The median follow-up time was 77 months (6 to 108 months).
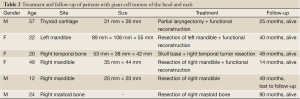
Full Table
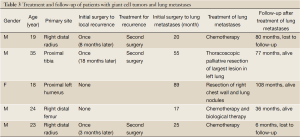
Full Table

Full Table
Case presentation
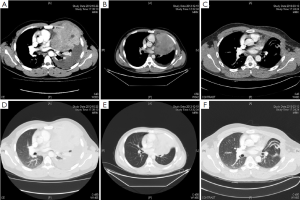
In November 2012, we treated one patient with pathological diagnosis of giant cell tumor of the left rib complicated with bloody pleural effusions. The patient was a 25-year-old man who complained of protrusion of the left anterior chest wall for three years, with intermittent pain for one year, which worsened for one month. The patient was aware of slight protrusion of the left anterior chest wall since 2009, which became more obvious with intermittent chest pain from September 2011. The left chest pain lasted for two hours in September 2012, followed by spontaneous remission, during which he felt difficulty in breathing and dizziness. In October 2012, bloody pleural effusion of 900 mL was extracted under ultrasonic imaging. He presented dry cough without phlegm. The CT scan is shown in Figure 1. He was otherwise healthy, without history of allergies or family history of cancer.
Physical examination
ECOG 0-1, no palpable enlargement of superficial lymph nodes; a mass was observed at the 2nd to 4th rib of the left anterior chest wall, 8 cm × 10 cm in size, visible over the skin surface. The mass was solid and hard, fixed, and without tenderness. No swelling and ulceration was observed on the surface.
CT scan
Expansion of the 3rd left rib was observed, and a soft tissue mass in size of 101 mm × 111 mm was seen. The boundaries were not clear, and enhanced signals were seen after enhancement. The lesion was not clearly separable from the intercostal muscles. Left pleural effusion and atelectasis of the majority of the left lung were found (Figure 1).
Auxiliary examination
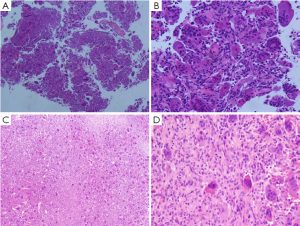
Two preoperative biopsies confirmed giant cell tumor (Figure 2); pleural fluid cytology revealed no tumor cells. Blood NSE was 25 ng/mL. No other abnormalities were observed.
Postoperative pathology
Giant cell tumor of bone. The mass was 12 cm × 10 cm × 8 cm in size, with a grayish yellow to taupe appearance on the section plane. The tumor was soft and encapsulated, closely adhesive to the ribs and chest wall. Microscopy: the tumor consisted of many short spindle cells, oval cells and multinucleated giant cells, with obvious hyperplasia of small stromal vessels (Figure 2).
Treatment
Two cycles of neoadjuvant chemotherapy with liposomal doxorubicin (60 mg d1) combined with ifosfamide (6 g/m2, aliquots for 3 days) were given on November 7 and November 30, 2012. CT scan showed that the bloody pleural effusions had almost disappeared (Figure 1). The left upper lung atelectasis improved, and the primary tumor efficacy evaluation was SD. On January 6, 2013, the patient underwent surgery. Intraoperative exploration found about 200 mL of yellow pleural effusions in the right chest cavity, and no pleural nodules were observed. The tumor was growing intrathoracically with a complete capsule, and part of it had adhered to the local pericardium and the left lung. The tumor was completely resected and chest wall reconstructed. The recovery was uneventful. The patient received a follow-up of two months (Figure 1). No recurrence was found during the postoperative follow-up.
Discussion
Giant cell tumors mainly occur in 20- to 40-year olds (8). The average age of this group was 28 years [0-60], where the proportion of men and women was 46/34 (1.35:1), with slightly more males, which was consistent with the report of Seethalakshmi (9). Giant cell tumors often occur at the ends of limb long bones. The incidence rates of lesions of limbs and head and neck in this group were 53% and 12%, respectively (9). Giant cell tumors are often associated with local invasion, recurrence and metastases, and neither age nor gender can predict the metastases (9). The incidence of recurrence is usually higher after curettage than after wide excision, and radiological and histopathological changes can not predict recurrence after curettage (10). Although P53 heterozygous deletions are now found in relapsed giant cell tumor (11), as well as high expression of P63 in the metastatic tissue (12) and the significant correlation between horseradish peroxidase and metastases (13), it is challenging to detect these indicators. Instead, serum Tartrate-Resistant Acid Phosphatase 5b (TRACP 5b), as a predictor of recurrence, is more convenient to measure (14). In this study, anyhow, we were not able to detect those makers routinegly.
The median age of onset of lung metastases in the present cohort was 23 years [18-35], with an incidence of 6.25% (5/80), which was consistent with other literature (4). Lung metastasis was most common in patients with local recurrence and distal radial tumors, with incidence rates of 54% and 38%, respectively (4), followed by those with tumors at the femur and ilium. All patients with lung metastases on presentation were those who had giant cell tumors at the limb long bones, including the distal radius (2/5, 40%) and those who had had local recurrence (3/5, 60%). Even occurring in non-limb long bones, giant cell tumors are likely to become locally invasive. For example, those occurring in the temporal bone may invade the skull base (15), which has a low recurrence rate after radical resection (16). However, the six patients with giant cell tumors at the head and face (mandible, temporal bone, mastoid bone) and neck (thyroid cartilage) surgically treated in our hospital were followed up for an average time of 42.6 months (14-90 months) and no local recurrence or metastasis was found.
Although lung metastases will occur if the primary lesion is left untreated (17), all lung metastases occurred after surgery of the primary lesions in the present cohort, with a median time interval of 25 months (17 to 89 months). Lung metastases usually occurred in the lung parenchyma, and endobronchial metastases were rare (18). In the present cohort, two patients received resection of the pulmonary metastases, which had consistent histopathological characteristics with the primary lesions and previous reports (9).
In general, even without any treatment, patients with giant cell tumors complicated with lung metastases can usually survive for a long term, with a favorable long-term survival rate (19). It is also shown in this study that the disease progression was very slow even with the presence of lung metastases. Thus, intense chemotherapy and radiotherapy is not recommended for patients with giant cell tumors and lung metastases (9). Active, intensified treatment will bring certain risks, as one patient presented degree 4 neutropenia with pneumonia during chemotherapy, resulting in a short-term efficacy of SD. Moreover, the one patient who had had three sequential cycles of three chemotherapy regimens manifested shrinkage of bilateral lung lesions six months after the chemotherapy, and the shrinkage continued during the subsequent follow-up of two years and remained stable. Such favorable outcomes were not caused by chemotherapy. Instead of chemotherapy, Giant cell tumor cells are associated with high expression of RANKL (20), which is promoted by PTHrP (21). Clinical reports have shown that bisphosphonates may control and improve the symptoms of patients with iliac lesions in whom surgery is difficult and embolization has failed (22). One patient with giant cell tumor of the cervical vertebra had remission of the primary lesion after treatment with bisphosphonates alone (23). A phase II clinical study suggested that denosumab might become the RANKL-targeted medication for locally advanced and refractory giant cell tumors of bone, providing a new approach to the control of disease progression and symptoms (24). In addition, giant cell tumors can also express some angiogenic growth factors. Based on clinical observations, a five-year-old patient with giant cell tumor of the palatine bone has been successfully treated with IFN-α as an anti-angiogenic agent (25). Another study has also been shown that IFN-α treatment is effective against giant cell tumors (26). In the era of targeted therapy, one patient of giant cell tumor treated with pazopanib combined with lapatinib has achieved PR (27).
Regarding surgical resection of lung metastases, it is generally believed that in the event of early detection of lung metastases during follow-up, surgical resection may prevent respiratory failure as a result of the progression of lung lesions. The effects of postoperative adjuvant chemotherapy and chemotherapy for lung metastases are unclear. Considering that giant cell tumors are borderline tumors, most reports suggest that they are not responsive to chemotherapy even after the appearance of the lung metastases. One patient with giant cell tumor of the metacarpal bone and multiple lung metastases was reported to achieve almost CR after chemotherapy with ADM + DDP (28). In the present cohort, three patients with giant cell tumors and lung metastases received chemotherapy regimens including CAP, MAID, IFO+VP-16, and BLM + CBP + ACD-T, with a median number of cycles of six [5-8], in which two patients undergoing CAP therapy had PR and PD, respectively, and other patients had SD or PD. Despite the failure of chemotherapy, two of the three patients presented long-term survival.
The role of neoadjuvant therapy in the treatment of locally unresectable giant cell tumors also has not been recognized. In our study, one 15-year-old boy with giant cell tumor of the cervical odontoid process who was treated with chemotherapy followed by surgical resection was reported to be free of recurrence for ten years during follow-up (29). We also treated a 25-year-old patient with giant cell tumor and large amount of bloody pleural effusion on presentation, so that surgery was successfully performed. After two cycles of preoperative chemotherapy with IFO + liposomal doxorubicin, the left pleural effusion was significantly reduced, and lung re-expansion was achieved. Though the efficacy evaluation for the primary tumor was SD, the lesion of the rib was completely removed and the chest wall reconstructed. During surgery, the pericardial and pleural effusions were yellow, and the cellular pathological finding was negative. It is shown that neoadjuvant chemotherapy is effective for this particular patient with bloody pleural effusion due to giant cell tumor.
Acknowledgements
This study was funded by Guangdong Medical Science and Technology Research Fund (A2011199) and Wu Jieping Medical Foundation Clinical Research and Special Assistance Funds (320.6750.1308).
Disclosure: The authors declare no conflict of interest.
References
- Campanacci M, Baldini N, Boriani S, et al. Giant-cell tumor of bone. J Bone Joint Surg Am 1987;69:106-14. [PubMed]
- Klenke FM, Wenger DE, Inwards CY, et al. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res 2011;469:1181-7. [PubMed]
- Gupta R, Seethalakshmi V, Jambhekar NA, et al. Clinicopathologic profile of 470 giant cell tumors of bone from a cancer hospital in western India. Ann Diagn Pathol 2008;12:239-48. [PubMed]
- Tubbs WS, Brown LR, Beabout JW, et al. Benign giant-cell tumor of bone with pulmonary metastases: clinical findings and radiologic appearance of metastases in 13 cases. AJR Am J Roentgenol 1992;158:331-4. [PubMed]
- Connell D, Munk PL, Lee MJ, et al. Giant cell tumor of bone with selective metastases to mediastinal lymph nodes. Skeletal Radiol 1998;27:341-5. [PubMed]
- Tyler W, Barrett T, Frassica F, et al. Skin metastasis from conventional giant cell tumor of bone: conceptual significance. Skeletal Radiol 2002;31:166-70. [PubMed]
- Alacacioğlu A, Bengi G, Oztop I, et al. Metastasis of giant cell tumor to the breast: case report and review of the literature. Tumori 2006;92:351-3. [PubMed]
- Curilovic A, Eich GF, Stallmach T. Giant cell tumor in the skull of a 9-year-old child: immunohistochemistry to confirm a diagnosis rare for age and site. Pediatr Pathol Lab Med 1995;15:769-79. [PubMed]
- Viswanathan S, Jambhekar NA. Metastatic giant cell tumor of bone: are there associated factors and best treatment modalities? Clin Orthop Relat Res 2010;468:827-33. [PubMed]
- Chanchairujira K, Jiranantanakorn T, Phimolsarnti R, et al. Factors of local recurrence of giant cell tumor of long bone after treatment: plain radiographs, pathology and surgical procedures. J Med Assoc Thai 2011;94:1230-7. [PubMed]
- Saito T, Mitomi H, Suehara Y, et al. A case of de novo secondary malignant giant-cell tumor of bone with loss of heterozygosity of p53 gene that transformed within a short-term follow-up. Pathol Res Pract 2011;207:664-9. [PubMed]
- Yanagisawa M, Kakizaki H, Okada K, et al. p63 as a prognostic marker for giant cell tumor of bone. Ups J Med Sci 2013;118:23-8. [PubMed]
- Conti A, Rodriguez GC, Chiechi A, et al. Identification of potential biomarkers for giant cell tumor of bone using comparative proteomics analysis. Am J Pathol 2011;178:88-97. [PubMed]
- Shinozaki T, Saito K, Kobayashi T, et al. Tartrate-Resistant Acid Phosphatase 5b is a Useful Serum Marker for Diagnosis and Recurrence Detection of Giant Cell Tumor of Bone. Open Orthop J 2012;6:392-9. [PubMed]
- Iizuka T, Furukawa M, Ishii H, et al. Giant cell tumor of the temporal bone with direct invasion into the middle ear and skull base: a case report. Case Rep Otolaryngol 2012;2012:690148.
- Yip KM, Leung PC, Kumta SM. Giant cell tumor of bone. Clin Orthop Relat Res 1996;60-4. [PubMed]
- Rock M, Capanna R. The treatment of giant cell tumor of bone. In:Stauffer RN. eds. Advances in Operative Orthopaedics. Vol 1.St Louis, MO: Mosby-Year Book Inc,1993:367-90.
- Boghani A, Gayathri K, Ratnakar KS. Endobronchial metastasis from giant cell tumor of bone. Chest 1994;106:1599-601. [PubMed]
- Dominkus M, Ruggieri P, Bertoni F, et al. Histologically verified lung metastases in benign giant cell tumours--14 cases from a single institution. Int Orthop 2006;30:499-504. [PubMed]
- Morgan T, Atkins GJ, Trivett MK, et al. Molecular profiling of giant cell tumor of bone and the osteoclastic localization of ligand for receptor activator of nuclear factor kappaB. Am J Pathol 2005;167:117-28. [PubMed]
- Cowan RW, Singh G, Ghert M. PTHrP increases RANKL expression by stromal cells from giant cell tumor of bone. J Orthop Res 2012;30:877-84. [PubMed]
- Chaudhary P, Khadim H, Gajra A, et al. Bisphosphonate therapy is effective in the treatment of sacral giant cell tumor. Onkologie 2011;34:702-4. [PubMed]
- Gille O, Oliveira Bde A, Guerin P, et al. Regression of giant cell tumor of the cervical spine with bisphosphonate as single therapy. Spine (Phila Pa 1976) 2012;37:E396-9.
- Silk AW, Schuetze SM. Histology-specific therapy for advanced soft tissue sarcoma and benign connective tissue tumors. Curr Treat Options Oncol 2012;13:285-98. [PubMed]
- Kaban LB, Mulliken JB, Ezekowitz RA, et al. Antiangiogenic therapy of a recurrent giant cell tumor of the mandible with interferon alfa-2a. Pediatrics 1999;103:1145-9. [PubMed]
- Kaban LB, Troulis MJ, Ebb D, et al. Antiangiogenic therapy with interferon alpha for giant cell lesions of the jaws. J Oral Maxillofac Surg 2002;60:1103-11; discussion 1111-3. [PubMed]
- de Jonge MJ, Hamberg P, Verweij J, et al. Phase I and pharmacokinetic study of pazopanib and lapatinib combination therapy in patients with advanced solid tumors. Invest New Drugs 2013;31:751-9. [PubMed]
- Moon JC, Kim SR, Chung MJ, et al. Multiple pulmonary metastases from giant cell tumor of a hand. Am J Med Sci 2012;343:171-3. [PubMed]
- Shirzadi A, Drazin D, Bannykh S, et al. Giant cell tumor of the odontoid in an adolescent male: radiation, chemotherapy, and resection for recurrence with 10-year follow-up. J Neurosurg Pediatr 2011;8:367-71. [PubMed]


