Synergistic inhibitory effect of wogonin and low-dose paclitaxel on gastric cancer cells and tumor xenografts
Introduction
Gastric cancer is the fourth most common malignant tumor in the world. The mortality from gastric cancer is preceded only by that of lung cancer. Approximately 40% of patients with gastric cancer miss the opportunity to undergo radical curative surgery at the time of diagnosis, while 50% of patients experience recurrence and metastasis after surgery (1). Therefore, chemotherapy plays an important role in the treatment of this cancer.
Cisplatin (CDDP) and paclitaxel (PTX) are frequently used in the systemic chemotherapy of patients with gastric cancer, and combination chemotherapy with two or three drugs is common with superiority in the first- or second-line treatment of gastric cancer (2). Although great achievements have been made in the treatment of gastric cancer, the response rate of combination chemotherapy is rarely over 50% (3). However, the toxicity of combination chemotherapy is greater than that of single drugs (4). Thus, the development of optimal chemotherapeutic regimens with high efficacy and low toxicity is necessary.
Wogonin (WOG) is a flavonoid extracted from the root of Scutellaria baicalensis Georgi. It is a phytochemical that has been widely used in the treatment of allergy, inflammation, hypertension, and cardiovascular diseases (5). Recently, in vitro and in vivo experiments have demonstrated that WOG can inhibit the growth of malignant tumors such as bladder cancer (6), myelogenous leukemia (7), hepatocellular carcinoma (8), gastric cancer (9), and glial tumors (10) through inducing Ca2+-dependent apoptosis (11), attenuation of NF-κB activity (12), and enhancement of the expression of p53 up-regulated modulator of apoptosis (PUMA), all of which could mediate its cytotoxicity (13).
Importantly, WOG inhibits the growth of malignant tumor cells but does not influence the proliferation of normal cells such as epithelial and peripheral blood cells (14), and prostate cells (13). Accordingly, regimens combining WOG and common chemotherapeutic drugs may achieve better efficacy with lower toxicity levels. Zhao et al. have previously reported that WOG can strengthen the efficacy of low doses of the chemotherapy drug fluorouracil without increasing toxicity in a nude mouse model of gastric cancer (9). However, there have been no investigations related to the effects of combining WOG and other common chemotherapeutic drugs such as CDDP and PTX in gastric cancer. Therefore, this study was conducted to investigate whether WOG synergizes the effects of either CDDP or PTX in gastric cancer in vitro and in vivo.
Materials and methods
Drugs and cell culture
WOG (kindly provided by Professor Qinglong Guo, China Pharmaceutical University), CDDP (Qilu Pharmaceutical Co., Ltd., China) and PTX (Hainanhaiyao Co., Ltd., China) were freshly prepared using sterile physiological saline before each use.
Human gastric cancer BGC-823, MGC-803, and MKN-45 cell lines (kindly provided by Professor Youyong Lv, Peking University Cancer Hospital & Institute) and HGC-27 cell line (purchased from the Shanghai Institute for Biological Sciences, CAS) were maintained in RMPI-1640 medium (Gibco) with 10% heat-inactivated fetal bovine serum (Gibco) under a humidified atmosphere of 5% CO2 and 95% air at 37 °C.
Cell proliferation assay
Cell proliferation was analyzed by CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS assay, Promega) in three replicates according to the manufacturer’s instructions. Briefly, cells (5,000/well) were grown in RPMI-1640 medium (100 µL/well) in 96-well plates and exposed to various concentrations of the experimental drug. After 48 h of drug exposure, 20 µL of MTS was added to the medium and cells were incubated in the CO2 incubator at 37 °C for 2-4 h. The absorbance was then read at 490 nm by a plate reader. Absorbance at 490 nm was directly proportional to the viable cell number in culture (15) and thus could be used to determine relative growth using the following calculation: [(Atreated–Azero)/(Acontrol–Azero)] ×100%. The Atreated reflected the living cell number in treated wells, the Acontrol depicted the living cell number of untreated wells, and the Azero reflected background absorbance, which is subtracted to correct the error.
Assessment of combination effects of two drugs
Based on the median effect method of Chou-Talalay (16), the IC50 values of individual drugs was obtained. The combination index (CI) was calculated by the following formula (17): CI = (D)1/(DX)1 + (D)2/(DX)2 + [k(D)1(D)2/(DX)1(DX)2], where k =0 for mutually exclusive drugs; (D)1 and (D)2 were the concentration of the separate drugs in their combination; (DX)1 and (DX)2 were individual drug concentrations that result in a cell inhibition ratio of X%. These concentrations were calculated by the formula: D = Dm [Fa/(1- Fa)]1/m, where Dm is the concentration required to attain an inhibition of 50%, fraction affected (Fa) represents the respective proliferation inhibition parameters for the separate drugs (e.g., an Fa of 0.5 is a proliferation inhibition of 50%), and m is the slope of the median effect plot. CI >1, CI =1, and CI <1 represent antagonism, additive effect, and synergism, respectively. Then, effects of the combination were displayed using forms of Fa-CI plots, which were the CI versus Fa and showed the evolution of drug interactions (synergism, antagonism, additive effects) (18).
Detection of cell apoptosis
Cells grown on coverslips in 12-well plates were exposed to individual drugs or two drug simultaneously for 48 h, then incubated with Hoechst 33258 (Hoechst Staining Kit, Beyotime, China). Fluorescence microscopy was used to observe cell shape captured from six random visual fields. The ratio of apoptotic cells to total cell number was calculated.
Xenograft model in nude mice
BGC-823 cells (1×106) suspended in phosphate buffered saline (0.1 mL) were subcutaneously injected into the right oxter of 6-week-old female BALB/c athymic nu/nu mice (Vital River, China). Seven days after injection, when tumor volumes were about 50 mm3, all mice (n=50) were randomly divided into 10 groups (n=5 per group) and treated intraperitoneally with different drugs as follows: control group (physiological saline, once a day for 2 weeks); WOG 60 mg/kg group (once a day for 2 weeks); PTX 10 mg/kg group (once a week for 2 weeks); PTX 20 mg/kg group (once a week for 2 weeks); CDDP 3 mg/kg group (once a week for 2 weeks); CDDP 6 mg/kg group (once a week for 2 weeks); WOG (60 mg/kg) plus PTX (10 mg/kg) group; WOG (60 mg/kg) plus PTX (20 mg/kg) group; WOG (60 mg/kg) plus CDDP (3 mg/kg) group; and WOG (60 mg/kg) plus CDDP (6 mg/kg) group. The length and width of tumors as well as mouse weight were measured twice per week from day 0 to 16, where day 0 was the day of initial treatment and day 16 was the day of sacrifice. Tumor volume was calculated by the formula V = L × W2 × π/6 (V, volume; L, length; W, width of tumor).
Statistical analysis
We performed statistical analysis using the Student’s t-test with SPSS software (SPSS Inc., Chicago, IL, USA). The statistical significance of the data was accepted when the P value was less than 0.05.
Results
WOG, CDDP, and PTX inhibit growth of gastric cancer cells in a dose-dependent manner
The effect of each drug on gastric cancer cell growth was first tested to evaluate cell-line-specific sensitivity. The IC50 values for WOG, CDDP, and PTX in BGC-823, MGC-803, MKN-45, and HGC-27 cell lines are shown in Table 1. The data demonstrated that the growth of all cell lines was inhibited by any of the drugs used independently in a dose-dependent manner, although different cells display different sensitivity to the same drug (Figure 1). Compared with any individual drug, the growth of cells treated with a combination of WOG and either CDDP or PTX was further inhibited in a dose-dependent manner (Figure 1).
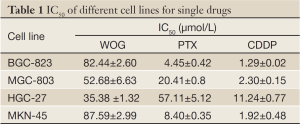
Full Table

Analysis of synergistic effects between WOG and CDDP/PTX in vitro
In BGC-823 and MGC-803 cell lines, WOG and CDDP induced significant synergistic growth inhibition (CI <1). While in HGC-27 and MKN-45 cell lines, WOG and CDDP had a synergistic inhibitory effect when Fa values were >0.65 and >0.1, respectively (Figure 2A). In BGC-823, MGC-803, HGC-27, and MKN-45 cell lines, synergism between WOG and PTX was seen when Fa values were <0.45, <0.9, <0.85, and <0.6, respectively (Figure 2B), suggesting that there might be antagonism between WOG and high-dose PTX.

Table 2 summarizes the values of CI and the concentration of the individual drugs in their combination when Fa =0.5. In BGC-823 cell lines, WOG plus PTX showed antagonism (CI >1), which was also observed for WOG plus CDDP in HGC-27 cell lines. In the combination of WOG and CDDP, the concentrations of WOG were 33.75%, 22.53%, and 26.54% of the WOG IC50, while the concentrations of CDDP were 42.48%, 28.83%, and 9.89% of the IC50 of CDDP in BGC-823, MGC-803, and MKN-45 cell lines, respectively. In the combination of WOG and PTX, the concentrations of WOG were 31.23%, 34.48%, and 53.49% of the WOG IC50, while the concentrations of PTX were 29.03%, 39.12%, and 33.08% of the PTX IC50 in MGC-803, HGC-27, and MKN-45 cell lines, respectively.
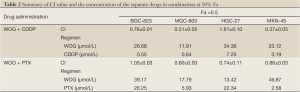
Full Table
Apoptosis is induced by WOG and CDDP/PTX
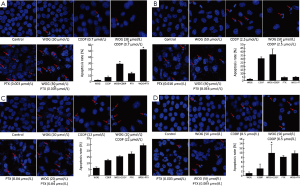
We chose drug concentrations that had a synergistic effect when used in combination therapy for follow-up experiments. First, apoptosis was evaluated in BGC-823, MGC-803, HGC-27, and MKN-45 cells that were exposed to single drugs or the combination treatments for 48 h. The cells were stained and evaluated for nuclear shape under an inverted fluorescence microscope (Figure 3). While untreated cells displayed evenly dispersed blue fluorescence within their nuclei, treated cells showed chromatin condensation or dense staining fragmentation called apoptotic bodies, which implied an early apoptotic event (19) (Figure 3). Meanwhile, the total number of cells in the single-drug-treated groups decreased compared with untreated groups. Importantly, a more obviously reduction was observed in combination groups. This was particularly more remarkable in the BGC-823 cell line (P<0.05, Figure 3A). However, there were similar trends in the other three cell lines (P>0.05). These results suggest that the combination of WOG and either CDDP or PTX could enhance the induction of apoptosis in gastric cancer cells.
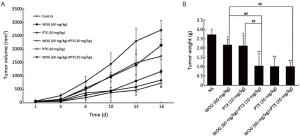
Inhibitory effect of drug combination on growth of BGC-823 tumor xenografts
Because WOG combined with PTX/CDDP significantly inhibited the growth of different gastric cancer cell lines, the effect of combination treatment on the growth of gastric cancer cell line-derived tumor xenografts in nude mice was explored.
The effect of combining WOG with CDDP was first tested. While there was no significant difference in tumor volumes before treatment (P>0.05), after treatment all five mice exhibited poor mental state and inactivity. In the next 2-3 d, mice became thinner and their skin lost luster, and they soon all died. To counteract the toxicity, we reduced the dose of CDDP by half, but the result was not changed. In contrast, the above situation did not happen in groups where mice were treated with either WOG or CDDP independently. These observations suggest that WOG and CDDP cannot be used together because of much greater toxicity than single agents.
In the study of treatment with a combination of WOG and PTX, treated groups, either single drugs or the combination, all showed an inhibitory effect on the growth of BGC-823 xenografts when compared with the non-treated control group. The combination treatment groups displayed even more obvious inhibition than corresponding single-drug groups. By the 16th day after the initial treatments, the mean tumor volume of the WOG plus PTX (10 mg/kg) group was much smaller than that of either the PTX-alone (10 mg/kg) or WOG-alone groups (1,171 mm3, 1,740 mm3, and 2,148 mm3, respectively) (P<0.05, Figure 4A). A similar trend was demonstrated among the WOG plus PTX (20 mg/kg) group, and the PTX-alone (20 mg/kg) or WOG-alone groups (P>0.05, Figure 4A). The mean tumor weights demonstrated that the combination treatments were more efficient than single drugs (Figure 4B). Indeed, in the WOG plus PTX (10 mg/kg) group, the inhibitory ratio (IR) of tumor weight was 61.58% while it was 20.29% and 22.28% in the WOG-alone and PTX-alone (10 mg/kg) groups, respectively. Interestingly, one mouse treated with PTX (20 mg/kg) presented with hemiplegia, which is an indicator of neurotoxicity. No weight loss was observed before and after treatments (Table 3).
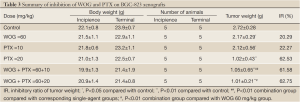
Full Table
Discussion
In China, the incidence and mortality of gastric cancer are twice that of the world average (20). For patients with advanced gastric cancer, chemotherapy plays an important role. The most desirable chemotherapeutic schedule aims to achieve a higher response rate, reduced toxicity, and delayed appearance of drug resistance. To date, the efficacy of existing chemotherapeutic drugs has not been satisfactory. Thus, the development of strategies that increase their anticancer activity is urgently needed. WOG is a promising new finding for anticancer therapy because it induces cytotoxic effects in many cancer cell lines and also animal tumor models, but, interestingly, WOG exhibits little toxicity towards normal tissues (21). Its main mechanism of action involves prolonged activation PLCr1 via H2O2 signaling, which leads to sustained elevation of cytosolic Ca2+ in malignant, but not normal, cells, and this subsequent Ca2+ overload leads to disruption of the mitochondrial membrane and cell death. Because of differences in mechanisms of action and toxicity profiles, the combination of WOG with conventional chemotherapy drugs boasts enormous clinical potential.
Our study investigated the antitumor effects of WOG combined with either of two chemotherapy drugs, PTX and CDDP, on different gastric cancer cell lines in vitro and on BGC-823 xenografts in vivo. As previously reported (9), treatment with any of the drugs alone inhibited the growth of gastric cancer cells in a dose-dependent manner. According to the IC50 values, the HGC-27 cell line was the most sensitive to WOG, while the BGC-823 cell line was the most sensitive to CDDP and PTX.
Using a fixed ratio of WOG to PTX/CDDP (WOG IC50:PTX/CDDP IC50 =1:1), we found that combination treatments had higher inhibitory rates on proliferation than monotherapies, within a certain range of concentration, on the four cell lines tested. This suggested that combination treatments were more efficacious than monotherapies.
The median-effect analysis method of Chou and Talalay is an emerging approach to evaluate combination effects of chemotherapeutic drugs in vitro. It can judge whether or not the interaction of drugs is synergistic or dynamic (16). According to this method, the combination of WOG and CDDP/PTX in the four cell lines tested all showed synergism in different ranges of Fa. For most of the cell lines, the dose required for drugs when used in combination treatments was lower than their IC50. These results demonstrate that identical effects could be acquired in combination therapies with lower doses of each drug compared with monotherapies. Therefore, individual toxicities associated with higher doses could be reduced. All these results provide evidence supporting the use of WOG in combination with chemotherapy drugs for clinical application.
As for a mechanism of action, WOG has been shown to induce extensive apoptosis in many malignant tumors such as hepatocellular carcinoma (22), ovarian cancer, prostate cancer (13), and murine sarcoma (23). Thus, triggering apoptosis is considered one of the mechanisms used by WOG to execute its anti-tumor effects. Indeed, Lee has reported that WOG increases expression of P53, a tumor suppressor protein, and its target genes, p21, p27, and PUMA, to promote cell apoptosis (13). Zhao has also demonstrated that WOG induces apoptosis by inhibiting the activity of NF-κB (9), as well as that of anti-apoptotic proteins such as Bcl-2 and Bcl-x. In our study, Hoechst staining revealed a more obvious apoptotic phenotype in cells receiving combination therapies than in those treated with monotherapies, especially in the BGC-823 cell line. Accordingly, apoptotic rates were highest in the combination groups in comparison with the single-drug or no-treatment control groups. These results suggest that further stimulating apoptosis is one way by which combination therapies can attain better efficacy.
To date, the effect of combining WOG and CDDP/PTX in gastric cancer cells has not been reported. Lee has previously found that compared with CDDP alone, the combination of WOG and CDDP did not enhance inhibitory effects in Jurkat and HL-60 cells (24). However, in our studies, the efficacy of combination treatments was significantly better than that of CDDP alone. These disparate findings may be because of metabolic differences critical to WOG and/or CDDP sensitivity among different cell lines (25). It has also been reported that WOG inhibits P-glycoprotein, one of the ABC transporter family proteins (26). Thus, it has been proposed that combination treatments exhibit synergistic effects probably because WOG can decrease the excretion of chemotherapeutic drugs by suppressing P-glycoprotein.
In this study, WOG combined with CDDP/PTX demonstrated significant anti-tumor activity in vitro. However, although the in vitro results provide information about cytotoxicity, the usefulness of these experiments is limited for evaluating the efficacy of drugs (25). Thus exploration in vivo was encouraged. To this end, WOG and PTX showed inhibition when used separately on both BGC-823 xenografts and MKN-45 xenografts (data not shown). However, combination of WOG and CDDP displayed toxic synergism. Indeed, no mice in this combination group survived, while survival rates were not influenced in monotherapy groups. The lethality of WOG combined with CDDP was also apparent in MKN-45 xenografts (data not shown). It is well described that treatments that increase therapeutic efficacy in vitro have the potential for markedly enhanced toxicity in vivo because of accumulation of drugs. Moreover, metabolic differences between cells and animals may change the synergistic relationships of two drugs (25). These reasons may explain why the synergy of WOG and CDDP observed in vitro was not verified in animals. Thus, in summary, WOG and CDDP are not good partners in the treatment of gastric cancer.
WOG combined with low-dose PTX, on the other hand, obtained the same efficacy as with high-dose PTX, which was much better than that of low-dose PTX- or WOG-alone groups. The IR were more than 60% in both the WOG plus PTX (10 mg/kg) and PTX (20 mg/kg) groups, while it was about 20% in single-drug groups. The use of PTX in the clinic is limited by its side effects such as allergic reactions, hematological toxicity, and neurotoxicity. In our experiment, one of the five mice treated with PTX alone (20 mg/kg) presented with hemiplegia, which is indicative of neurotoxicity. In contrast, the combination of WOG and PTX was not related to any toxicity; indeed, no differences in body weight and survival rate were noted in mice of this treatment group. Instead, it was found that the combination of WOG and low-dose PTX has a satisfactory synergistic effect in vivo.
On the whole, this is the first study in which the inhibitory effects of WOG combined with CDDP/PTX on the growth of different gastric cancer cells and on BGC-823 xenografts were compared. Both combination treatments showed significant inhibitory effects in vitro, but only the combination of WOG and low-dose PTX was efficacious and safe when used for animal experiments. This study provides evidence supporting the potential clinical efficacy of combination treatment involving low-dose PTX and WOG to elicit a synergetic anti-tumor effect in gastric cancer.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Gao J, Lu M, Yu JW, et al. Thymidine Phosphorylase/β-tubulin III expressions predict the response in Chinese advanced gastric cancer patients receiving first-line capecitabine plus paclitaxel. BMC Cancer 2011;11:177. [PubMed]
- Wagner AD, Grothe W, Haerting J, et al. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006;24:2903-9. [PubMed]
- Sastre J, Garcia-Saenz JA, Diaz-Rubio E. Chemotherapy for gastric cancer. World J Gastroenterol 2006;12:204-13. [PubMed]
- Wagner AD, Unverzagt S, Grothe W, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2010;CD004064. [PubMed]
- Chi YS, Lim H, Park H, et al. Effects of wogonin, a plant flavone from Scutellaria radix, on skin inflammation: in vivo regulation of inflammation-associated gene expression. Biochem Pharmacol 2003;66:1271-8. [PubMed]
- Ikemoto S, Sugimura K, Yoshida N, et al. Antitumor effects of Scutellariae radix and its components baicalein, baicalin, and wogonin on bladder cancer cell lines. Urology 2000;55:951-5. [PubMed]
- Lee WR, Shen SC, Lin HY, et al. Wogonin and fisetin induce apoptosis in human promyeloleukemic cells, accompanied by a decrease of reactive oxygen species, and activation of caspase 3 and Ca(2+)-dependent endonuclease. Biochem Pharmacol 2002;63:225-36. [PubMed]
- Chang WH, Chen CH, Lu FJ. Different effects of baicalein, baicalin and wogonin on mitochondrial function, glutathione content and cell cycle progression in human hepatoma cell lines. Planta Med 2002;68:128-32. [PubMed]
- Zhao Q, Wang J, Zou MJ, et al. Wogonin potentiates the antitumor effects of low dose 5-fluorouracil against gastric cancer through induction of apoptosis by down-regulation of NF-kappaB and regulation of its metabolism. Toxicol Lett 2010;197:201-10. [PubMed]
- Kim H, Kim YS, Kim SY, et al. The plant flavonoid wogonin suppresses death of activated C6 rat glial cells by inhibiting nitric oxide production. Neurosci Lett 2001;309:67-71. [PubMed]
- Baumann S, Fas SC, Giaisi M, et al. Wogonin preferentially kills malignant lymphocytes and suppresses T-cell tumor growth by inducing PLCgamma1- and Ca2+-dependent apoptosis. Blood 2008;111:2354-63. [PubMed]
- Fas SC, Baumann S, Zhu JY, et al. Wogonin sensitizes resistant malignant cells to TNFalpha- and TRAIL-induced apoptosis. Blood 2006;108:3700-6. [PubMed]
- Lee DH, Kim C, Zhang L, et al. Role of p53, PUMA, and Bax in wogonin-induced apoptosis in human cancer cells. Biochem Pharmacol 2008;75:2020-33. [PubMed]
- Liu ZL, Tanaka S, Horigome H, et al. Induction of apoptosis in human lung fibroblasts and peripheral lymphocytes in vitro by Shosaiko-to derived phenolic metabolites. Biol Pharm Bull 2002;25:37-41. [PubMed]
- Liao B, Hu Y, Brewer G. RNA-binding protein insulin-like growth factor mRNA-binding protein 3 (IMP-3) promotes cell survival via insulin-like growth factor II signaling after ionizing radiation. J Biol Chem 2011;286:31145-52. [PubMed]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 1984;22:27-55. [PubMed]
- van Moorsel CJ, Veerman G, Vermorken JB, et al. Mechanisms of synergism between gemcitabine and cisplatin. Adv Exp Med Biol 1998;431:581-5. [PubMed]
- Chou TC. The mass-action law based algorithm for cost-effective approach for cancer drug discovery and development. Am J Cancer Res 2011;1:925-54. [PubMed]
- Yang Y, Yang L, You QD, et al. Differential apoptotic induction of gambogic acid, a novel anticancer natural product, on hepatoma cells and normal hepatocytes. Cancer Lett 2007;256:259-66. [PubMed]
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137-50. [PubMed]
- Himeji M, Ohtsuki T, Fukazawa H, et al. Difference of growth-inhibitory effect of Scutellaria baicalensis-producing flavonoid wogonin among human cancer cells and normal diploid cell. Cancer Lett 2007;245:269-74. [PubMed]
- Wang W, Guo Q, You Q, et al. Involvement of bax/bcl-2 in wogonin-induced apoptosis of human hepatoma cell line SMMC-7721. Anticancer Drugs 2006;17:797-805. [PubMed]
- Wang W, Guo QL, You QD, et al. The anticancer activities of wogonin in murine sarcoma S180 both in vitro and in vivo. Biol Pharm Bull 2006;29:1132-7. [PubMed]
- Lee E, Enomoto R, Suzuki C, et al. Wogonin, a plant flavone, potentiates etoposide-induced apoptosis in cancer cells. Ann N Y Acad Sci 2007;1095:521-6. [PubMed]
- Keane TE, Gingrich JR, Rosner G, et al. Combination versus single agent therapy in effecting complete therapeutic response in human bladder cancer: analysis of cisplatin and/or 5-fluorouracil in an in vivo survival model. Cancer Res 1994;54:475-81. [PubMed]
- Lee E, Enomoto R, Koshiba C, et al. Inhibition of P-glycoprotein by wogonin is involved with the potentiation of etoposide-induced apoptosis in cancer cells. Ann N Y Acad Sci 2009;1171:132-6. [PubMed]


