A novel PTEN gene promoter mutation and untypical Cowden syndrome
Introduction
Cowden syndrome (CS) is a multi-system disorder involving increased risks for a number of malignancies as well as benign hamatomatous overgrowth of various tissues (1,2). CS was first described in 1963 (3), and was named after the family in which it was reported. Approximately 40% to 60% of CS patients inherit the disease. The International Cowden Consortium developed the original diagnostic criteria. More than 80% of patients who strictly met these criteria were subsequently found to have a phosphatase and tensin homolog (PTEN) mutation.
The PTEN gene is located on chromosome subband 10q23.3 and codes for a major lipid phosphatase. It has a central role in regulating the phosphatidylinositol-3-kinase (PI3K) signal transduction cascade (4). It is now known to be critically important both during embryonic development and as a tumor suppressor in mature organisms. Inactivation of PTEN may be caused by germline mutations of exons or promoter (5). Germline mutations in PTEN gene occur in 85% of patients with CS (6). Teresi et al. had emphasized the importance of PTEN promoter nucleotide variations and their ability to lead to CS progression (7). In addition to screening for deletions involving PTEN, using sequence analysis of the PTEN promoter to identify PTEN mutations is currently one of the criteria of CS diagnosis.
In the present study, we sequenced the exons and the promoter of PTEN to determine the mutation of PTEN gene. We found mutations in the PTEN promoter, which might induce the dysfunction of PTEN.
Methods and materials
Patient characteristics
A 72-year-old woman was admitted to Peking Union Medical College Hospital (PUMCH) for an investigation of her personal history of multiple neoplasms. She suffered medullar carcinoma in the left breast without lymph node invasion at the age of 56. She underwent a modified radical mastectomy followed by taking Tamoxifen for 7 years without any signs of cancer relapse. She suffered colon tubulovillous carcinoma mosaicking with tubulovillous adenoma and severe atypical hyperplasia and papillary carcinoma in the right lobe of thyroid gland at the age of 69. In the following year, she was found to harbor a myoschwannoma at the right C3-4 intervertebral foramen. This benign tumor was again surgically removed concerning the possibility of spinal cord compression. A brief survey of diseases of her family members was performed.
Samples collection
Tissues of the breast cancer, colon cancer, thyroid cancer, and myoschwannoma were collected in operation when tumors were removed from the patient. A part of the tissues were paraffin-embedded for pathologic analysis. A part of tissues were snap-frozen with liquid nitrogen, and were stored in –80 °C refrigerator until further assay. Also the clinical characteristics of the patient were collected.
Immunohistochemical analysis
5-µm-thick paraffin-embedded tissue sections were deparaffinized with xylene and rehydrated with ethanol. Endogenous peroxidase was blocked with 0.3% H2O2. Antigen retrieval was performed in 0.1 mol/L sodium citrate buffer (pH 6.0) with a microwave. Samples were incubated with rabbit anti-human PTEN polyclonal antibody (ab31392, 1:1,000, Abcam, Cambridge, UK) at room temperature and detected with a horseradish peroxidase (HRP) conjugated compact polymer system, and 3,3'-diaminobenzidine (DAB) was used as the chromogen. Slides were counterstained with hematoxylin and mounted with depex. Photographs of immunohistochemically stained sections were taken by a camera mounted on a Keyence BZ-8000 digital microscope (Keyence, Osaka, Japan). Immunochemical staining was examined by two pathologists blinded to the origin of the sections independently.
DNA extraction and sequencing
Genomic DNA was isolated from 30 mg tissues using ChargeSwitch® gDNA Mini Tissue Kit following the producer’s instruction. To amplify 9 exons and promoter between –1,389 bp and –707 bp upstream of the translation start codon of PTEN, poly chain reaction(PCR) was performed using the primers listed in Table 1 with 5 µg genomic DNA as template. The PCR products were purified and sequenced with Sanger Sequencing which was performed by lifetechnology.
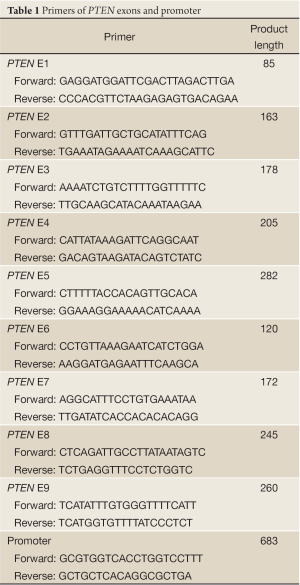
Full Table
Results
Outcomes of patient and pedigree of family
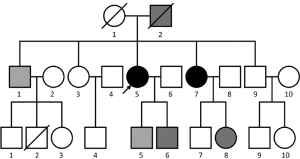
The patient still survived when the article was prepared. The pedigree of the family showed a relatively high incidence of malignancies in this family (Figure 1). The pedigree of the family showed that seven persons had suffered cancer. One person was the 1st generation, 3 persons were the 2nd generation and 3 persons were the 3rd generation. The types of cancers were showed in Figure 1.
Expression of PTEN in tumors
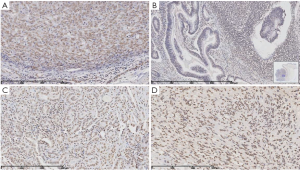
To investigate the expression of PTEN in the four surgically resected tumor tissues, immunohistochemical analysis was performed. In most slices, PTEN expressed normally (Figure 2A,C,D). A PTEN-negative region was found at the basal part of the colon tubulovillous carcinoma (Figure 2B).
Mutation of PTEN
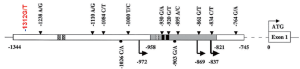
To further explore the mechanisms of PTEN expression in the malignance, we performed a series of analyses on PTEN gene. We sequenced 9 exons of PTEN, and found no mutation of the exons (data not shown). Then the PTEN promoter was sequenced with primers designed to amplify the region between –1,389 bp and –707 bp upstream of the translation start codon. Which included the full–length PTEN promoter between –1,344 bp and –745 bp. We examined the downstream effect of PTEN promoter variants (such as –861G/T, –853C/G, –834C/T, –798G/C, and –764G/A), which have been reported to be associated with CS. But we found no mutations of above promoter variants. A G>T mutation was found at –1,312 (Figure 3).
Discussion
CS is a rare autosomal dominant inherited disorder characterized by multiple tumor-like growths called hamartomas with an increased risk of certain forms of cancer (1). CS follows an autosomal dominant inheritance pattern in which a mutation happens in only one copy of the gene. Thus the descendants of the patients may suffer CS. The pedigree of the family may implicit this, but the limitation of the current study is that we could not get the DNA species and sequence the DNA of all members of the family. At least four genes, PTEN, SDHB, SDHD, and KLLN, have been identified in people with CS or Cowden-like syndrome. Most cases of CS and a small percentage of cases of Cowden-like syndrome result from mutations in PTEN gene (8). According to the International Cowden Consortium Operational Diagnostic Criteria, about 80% of patients with CS demonstrate germline PTEN mutations (9). In the current study, we found the dysfunction of PTEN, and the sequencing results showed the mutation of PTEN which located in the promoter.
PTEN has dual protein and lipid phosphatase activity, and its tumor suppressor activity is depend on its lipid phosphatase activity, which negatively regulates the PI3K-AKT-mTOR pathway (10). Dysfunction of PTEN protein results in disorder of signal pathway of PTEN/AKT (4). This may caused by mutation of exons or promoter (11). The mutations of exons down-regulate the expression of PTEN protein and contribute to PTEN-related diseases such as cancers (12,13). Reports have shown the roles of PTEN in breast tumor, gastric cancer and lung cancer (14-16). Germline mutations in PTEN have been described in a variety of rare syndromes that are collectively known as the PTEN-hamartoma tumor syndromes (PHTS). And CS is the best-described syndrome within PHTS (6). Patients with CS have an increased incidence of cancers of the breast, thyroid and endometrium, which correspond to sporadic tumor types that commonly exhibit somatic PTEN inactivation. PTEN is a constitutively expressed in normal cells (17). Zhou et al. have revealed a reduction in protein with PTEN promoter mutation-positive CS patients (18). In the present study, we found the patient have tubulovillous carcinoma, breast cancer and myoschwannoma. In this case, we did find a PTEN-negative region in the immunohistologically stained slices, which was located on the basal part of the colon tubulovillous adenoma. Subsequent sequencing revealed six mutations, five of which were located within the previously reported PTEN promoter region. These mutations may result in underexpression of PTEN, and increase cancer susceptibility. Teresi et al. have revealed that PTEN promoter was regulated by statins and SREBP (18). Sheng et al. have reported that a P53-binding sequence is located within the 599 bp fragment (–1,344/–745) in PTEN promoter (19). In the present study, we found the mutation of PTEN located in –1,312, which is the region of P53-binding sequence. We hypothesized that the mutation of this location may interfere the P53-induced PTEN expression, but this needs further investigation. And the successive incidence of tumors with germline mutations of PTEN needs further study.
Moreover, we should not ignore other possible explanation for the oncogenesis in this case, for instance, the germline KILLIN or PTEN methylation (20,21). As it is found recently that even subtle changes in expression of the PTEN tumor suppressor gene, such as hyper-methylation, can significantly increase cancer susceptibility in several organs, and may account for CS and Cowden-like syndrome (22). And the patients with germline variations in succinate dehydrogenase (SDH) genes also have higher thyroid and breast cancer prevalence in Cowden and Cowden-like syndrome. Studying PTEN in the continuum of rare syndromes provides insight into the role of PTEN in progression of rare disease and will inform targeted drug development.
In conclusion, the promoter of PTEN may turn off occasionally leading to the disorder of PTEN and the incidence of CS.
Acknowledgements
This work was supported by National Natural Science Foundation of China (30970623), International Science and Technology Cooperation Projects (2010DFA31840 and 2010DFB33720), and Beijing Natural Science Foundation (5112030).
Disclosure: The authors declare no conflict of interest.
References
- Gustafson S, Zbuk KM, Scacheri C, et al. Cowden syndrome. Semin Oncol 2007;34:428-34. [PubMed]
- Pilarski R. Cowden syndrome: a critical review of the clinical literature. J Genet Couns 2009;18:13-27. [PubMed]
- Uppal S, Mistry D, Coatesworth AP. Cowden disease: a review. Int J Clin Pract 2007;61:645-52. [PubMed]
- Lee JT, Shan J, Zhong J, et al. RFP-mediated ubiquitination of PTEN modulates its effect on AKT activation. Cell Res 2013;23:552-64. [PubMed]
- Marsh V, Davies EJ, Williams GT, et al. PTEN loss and KRAS activation cooperate in murine biliary tract malignancies. J Pathol 2013;230:165-73. [PubMed]
- Zbuk KM, Eng C. Cancer phenomics: RET and PTEN as illustrative models. Nature reviews Cancer 2007;7:35-45. [PubMed]
- Teresi RE, Zbuk KM, Pezzolesi MG, et al. Cowden syndrome-affected patients with PTEN promoter mutations demonstrate abnormal protein translation. Am J Hum Genet 2007;81:756-67. [PubMed]
- Fisher SB, Fisher KE, Maithel SK. Molecular targeted therapy for biliary tract malignancy: defining the target. Hepatobiliary Surg Nutr 2012;1:53-54.
- Podralska M, Nowakowska D, Steffen J, et al. First Polish Cowden syndrome patient with confirmed PTEN gene mutation. Arch Med Sci 2010;6:135-7. [PubMed]
- Sun D, Toan X, Zhang Y, et al. Mammalian target of rapamycin pathway inhibition enhances the effects of 5-aza-dC on suppressing cell proliferation in human gastric cancer cell lines. Sci China C Life sci 2008;51:640-7. [PubMed]
- Wang X, Jiang X. PTEN: a default gate-keeping tumor suppressor with a versatile tail. Cell Res 2008;18:807-16. [PubMed]
- Carracedo A, Alimonti A, Pandolfi PP. PTEN level in tumor suppression: how much is too little? Cancer Res 2011;71:629-33. [PubMed]
- Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nature reviews Cancer 2011;11:289-301. [PubMed]
- Wallace JA, Li F, Leone G, et al. PTEN in the breast tumor microenvironment: modeling tumor-stroma coevolution. Cancer Res 2011;71:1203-7. [PubMed]
- Feng S, Pan J, Wu Y, et al. Study on gastric cancer blood plasma based on surface- enhanced Raman spectroscopy combined with multivariate analysis. Sci China Life Sci 2011;54:828-34. [PubMed]
- Marino D, Colombi F, Ribero D, et al. Targeted agents: how can we improve the outcome in biliary tract cancer? Hepatobiliary Surg Nutr 2013;2:31-3.
- Zhang Z, Zhang X. Mechanical behavior of the erythrocyte in microvessel stenosis. Sci China Life Sci 2011;54:450-8. [PubMed]
- Zhou XP, Waite KA, Pilarski R, et al. Germline PTEN promoter mutations and deletions in Cowden/Bannayan-Riley-Ruvalcaba syndrome result in aberrant PTEN protein and dysregulation of the phosphoinositol-3-kinase/Akt pathway. Am J Hum Genet 2003;73:404-11. [PubMed]
- Sheng X, Koul D, Liu JL, et al. Promoter analysis of tumor suppressor gene PTEN: identification of minimum promoter region. Biochem Biophys Res Commun 2002;292:422-6. [PubMed]
- Bennett KL, Mester J, Eng C. Germline epigenetic regulation of KILLIN in Cowden and Cowden-like syndrome. JAMA 2010;304:2724-31. [PubMed]
- Lan F, Shi Y. Epigenetic regulation: methylation of histone and non-histone proteins. Sci China C Life Sci 2009;52:311-22. [PubMed]
- Yin L, Cai WJ, Liu CX, et al. Analysis of PTEN methylation patterns in soft tissue Sarcomas by massARRAY spectrometry. PLoS One 2013;8:e62971. [PubMed]


