Matrix metalloproteinase gene expressions might be oxidative stress targets in gastric cancer cell lines
Introduction
To live in homeostasis, every single organism must control the regulatory mechanism of itself against the changing conditions of the environment. From bacteria to mammals, oxidative stress has devastating effects which are the protein dysfunction, the lipid peroxidation, the breakdown of DNA, the change of the genetic code via mutations, and finally apoptosis (1,2).
Oxidative stress can trigger many cell signaling pathways to protect the normal cell life. Low levels of oxidative stress activate the transcription of genes encoding proteins that participate in the defense against oxidative injuries, oxidative damage repair mechanisms, and apoptosis (3). Nuclear accumulation of Nrf-2 and NF-κB causes transcriptional activation of antioxidant enzymes including superoxide dismutases, glutathione peroxidases and thioredoxin, respectively (3-6). If these first defense attempts are not sufficient to prevent oxidative stress-induced DNA damage, several other signaling processes such as base excision repair (7), cell cycle arrest at G1-S transition, S phase, and G2-M transitions (8) serve as protective mechanisms. In MCF-7 breast cancer cells, oxidative stress causes the expressions of CYGB, FOXM1, NOX5, NUDT1 and SEPP1 genes which can be affected differentially. Especially, the expression of FOXM1 may suppress cancer development and increases the effects of anticarcinogenic agents (9). In MDA-MB-231 breast cancer cells, oxidative stress responses of the cells are increased by NRP/B, a nuclear matrix protein, via the Nrf2 pathway. Nrf2 pathway associates with antioxidant response element (ARE) sourced detoxifying and antioxidant genes (10). Oxidative stress also regulates the role of β-catenin which is a key element of canonical Wnt pathway through binding and activating T cell factor (TCF) transcription factor. Under oxidative stress, the transcriptional activity of β-catenin/TCF complex is inhibited (11,12). FOXO, another transcription factor that is modulated by β-catenin, interacts with β-catenin in increased oxidative stress resulting with increased expression of anti-oxidant genes. FOXO can also inhibit the cell cycle progress under this condition because of its regulator role in G1 transition of the cell cycle (12). Additionally, HIF-1 and HIF-2, transcription factors that are responsible for the response of cells to hypoxia, directly bind to β-catenin and regulate the adaptation of cells to hypoxia (13).
The balance between pro-oxidants and antioxidants determines the extent of oxidative damage induced by reactive oxygen species (ROS). The shift from antioxidants to pro-oxidants in the tissue has been linked to increased risk of cancer (14,15). Chronic inflammation as a consequence of persistent oxidative stress has been linked to various steps involved in carcinogenesis, including cellular transformation, promotion, survival, proliferation, invasion, angiogenesis, and metastasis (16,17). Our insight in the molecular pathways involved in these biological responses to different oxygen radicals is still incomplete.
Matrix metalloproteinases (MMPs) are zinc-dependent proteins that are responsible for degrading extracellular matrix. Due to their capabilities, they can play important roles in growth, development and pathological processes. In carcinogenesis, they may provide suitable growth, invasion and finally metastasis conditions for tumor cells. Different MMP types can take action in different stages of carcinogenesis. These all depend on the cell environment and the needs of tumor progression (18).
Gastric cancer is the seventh most common cancer and the second most common cause of cancer-related death worldwide (19). The gastric epithelium is continuously exposed to toxic ROS within the gastric lumen due to ingested food and cigarette smoke and inflammation due to Helicobacter pylori infection. The dynamic balance between cell proliferation and apoptosis is essential for maintaining mucosal homeostasis. Decreased apoptosis as well as increased proliferation may favor the carcinogenic process. Prolonged survival of abnormal cells can support the accumulation of sequential genetic mutations, changes in gene expression profiles and protein structure and function which can result in tumor promotion (20-24). Consequently, the link between oxidative stress and gastric cancer cannot be ignored. However, the molecular mechanisms behind it are still not clear.
In this study, the changes in the expression levels of MMP genes were analyzed following H2O2 exposure of gastric carcinoma MKN-45 and 23132/87 cell lines to understand whether a relation exists between oxidative stress formation in gastric cancer cells and the expressions of MMP genes.
Materials and methods
Cell culture
Human gastric adenocarcinoma cell lines, MKN-45 (DSMZ ACC409) and 23132/87 (DSMZ ACC201), were purchased from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ) (Braunschweig, Germany). The cells were cultured in a standard RPMI-1640 (Biochrom, Berlin, Germany) medium supplemented with 10% (V/V) fetal bovine serum (FBS) and 1% (V/V) penicillin and streptomysin (10,000 µg/mL, Biochrom) in a humidified chamber at 37 °C in the presence of 5% CO2.
H2O2 treatment of cell lines
The cells were plated at a density of 3×103-5×103 cells per well and cultured in the standard medium overnight. The medium was aspirated and washed with phosphate buffered saline (PBS); the cells were starved in the culture medium supplemented with 0.01% FBS overnight and they were treated with indicated concentrations of H2O2 ranging from 50-200 µmol/L for indicated time points. The control cells were only starved and were not exposed to H2O2. The morphological changes were analyzed under microscope (Axiovert 40 CFL, Zeiss, Jena, Germany).
Determination of 8-hydroxydeoxyguanosine (8-OHdG) in cell lines
MKN-45 and 23132/87 cell lines that were exposed to H2O2 ranging from 50 to 200 µmol/L for 24 h were fixed with 4% paraformaldehyde for 15 min at room temperature. After the fixation, the presence of 8-OHdG in the cells was determined using DakoCytomation LSAB2 System-HRP kit (Dako, Hamburg, Germany). Mouse monoclonal 8-OHdG antibody (Cosmobio, Tokyo, Japan) was used as primary antibody. The sections were treated for 30 min at room temperature first with biotinylated goat anti-rabbit and goat anti-mouse IgG (Cosmobio) and then with streptavidin-horseradish peroxidase (HRP) solution. Between incubations, the sections were washed with 1× PBS (pH 7.4). 3-3'-diaminobenzidine tetrahydrochloride (DAB) (Sigma Chemical Co., St. Louis, Mo., USA) was used as HRP substrate. The sections were incubated with substrate-chromogen solution for 5 min and counterstaining was performed with Mayer’s hematoxylin to visualize the nucleus (Zeiss, Axiovert 40 CFL).
Reverse transcription-polymerase chain reaction (RT-PCR)
mRNA transcripts encoding MMP-1, MMP-3, MMP-7, MMP-9, MMP-10, MMP-11, MMP-12, MMP-14, MMP-15, MMP-17, MMP-23, MMP-28, β-catenin, and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) genes were determined by RT-PCR. Samples were homogenized to break them up. RNA was extracted from the cell lines using NucleoSpin RNA II kit (Macherey-Nagel, Oensingen, Switzerland). RNA concentration and purity of each extract were determined by A260/A280 absorptions using Shimadzu UV-1202 spectrophotometer. RNA extracts were stored at –80 °C for long-term use. cDNA was synthesized using oligo(dT)18 primer according to the manual of RevertAidTM First Strand cDNA Synthesis Kit (Fermentas, Vilnius, Lithuania). Primers for amplification of selected MMPs, β-catenin, and GAPDH and the PCR conditions are shown in Table 1. To determine the optimum number of cycles required for the amplification of these genes, an aliquot of first strand cDNA was amplified with the respective primers using an increasing number of PCR cycles (20-35). Initial denaturation was performed at 94 °C for 4 min. The subsequent cycling programs consisted of denaturation at 95 °C for 30 s, annealing for 30 s (annealing temperatures, see Table 1), and extension at 72 °C for 30 s, followed by a final extension at 72 °C for 5 min. The amplified products were separated on a 2% agarose gel, stained with ethidium bromide, and photographed using Gel Doc 2000 imaging system (Bio-Rad, Hercules, CA). PCR reactions in which the first strand cDNA was omitted served as negative controls. To avoid technical error, each PCR experiment was repeated three times.
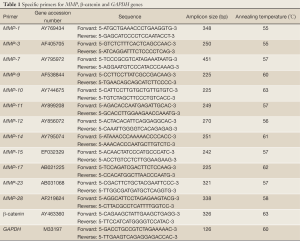
Full Table
Determination of oxidative stress in cell lines
Oxidative stress in the cell lines was assessed using an oxidative stress marker, 2',7'-dichlorofluorescein diacetate (DCFH-DA) (25). The non-fluorescent DCFH-DA (Sigma) is a cell-permeable compound that can enter into the cells, where it is deacetylated and entrapped as 2',7'-dichlorofluorescein (DCFH) of which by ROS produces a highly fluorescent product, DCF, which can be visualized under a fluorescent microscope. DCFH-DA is freshly prepared in 10 mmol/L HEPES (pH 7.5), 10 mmol/L glucose and 1 μmol/L DCFDA (dissolved in methanol) in PBS. MKN-45 and 23132/87 cells were treated with 200 μmol/L H2O2 for 24 h and after this time point, the medium was aspirated, and the cells were washed two times with PBS, and incubated with DCFH-DA solution at 37 °C for 15 min, and then were washed two times with PBS and observed under fluorescent microscope (Axio-skop, Zeiss, Jena, Germany). The live cells that were under oxidative stress were counted in five 20× fields per culture (typically 70-100 cells/20× field), and the percentage of the cells under oxidative stress in each culture was calculated.
Removal of stress with caffeic acid phenethyl ester (CAPE) in cell lines
The generated oxidative stress with 200 μmol/L H2O2 was eliminated with CAPE. Both of the cell lines were grown to 70% confluency in RPMI medium supplemented with 10% FBS. Three hours after the addition of 3 μg/mL CAPE (Sigma) to the growth medium, 200 μmol/L H2O2 was applied to the cells. After 24 h of incubation of the cells in CO2 incubator, the presence of oxidative stress in the live cells was demonstrated using DCFH as mentioned above. CAPE-untreated controls with only 200 μmol/L H2O2 exposure were also used in the experiment.
Quantitative real-time PCR
Total RNA was isolated and cDNA was synthesized from the cell lines using RNA extraction kit (NucleoSpin RNA II, Macherey-Nagel) and cDNA synthesis kit (RevertAid™ First Strand cDNA Synthesis Kit, Fermentas), respectively, according to the manufacturer’s instructions. Associated genes, their primers and annealing temperatures are listed in Table 1. As an internal control, the GAPDH mRNA was also amplified in PCR reactions. The reactions were carried out with 2 µL cDNA template in a total volume of 25 µL, containing 1× iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) with primers for whole genes in a Rotor-Gene real-time PCR instrument (Corbett Research, Sydney, Australia). After initial denaturation at 95 °C for 10 min, 40 cycles of denaturation at 95 °C for 10 s, annealing at different temperatures from 55 to 63 °C (see Table 1) for 25 s and extension at 72 °C for 25 s were carried out. Finally, melting analysis was performed in the temperature range of 55 to 95 °C to verify product homogeneity. Real-time PCR reactions were carried out in triplicate for each sample as a technical replicate. Each cDNA sample was tested in three different reactions with three technical replicates and negative controls. Three biological replications have been performed for each transcript in order to determine whether there are significant differences in the expressions at the different time points.
Statistical analysis
Each of the data is the mean of three independent values and all data are expressed as 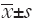 . Student’s t-test is used in order to assess whether the means of mRNA expression rates of untreated control cells and treated cells are statistically different from each other by using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
. Student’s t-test is used in order to assess whether the means of mRNA expression rates of untreated control cells and treated cells are statistically different from each other by using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
Results
Morphological changes of cell lines
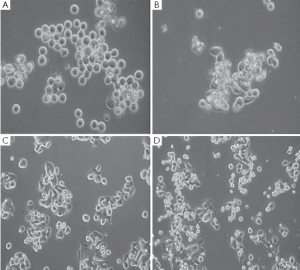
Gastric cancer cell lines, MKN-45 and 23132/87, exhibited a remarkable change in their morphologies and loss of surface attachment following H2O2 exposure when they were compared with their H2O2-untreated controls as shown by inverted light microscopic views with the presence of 8-OHdG (data not shown). The unexposed control cells exhibited distinctive cell shape having regular plasma membrane boundaries with apparent cytoplasm and nucleus whereas H2O2 exposure resulted in irregular cell membrane with nuclear and cytoplasmic shrinking, and chromatin condensation in the cell lines (Figure 1).
Determination of MMP expressions
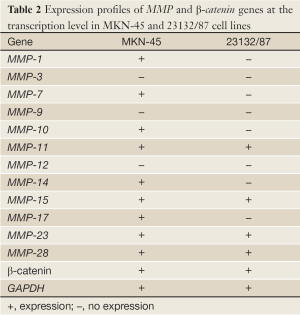
MKN-45 and 23132/87 cell lines showed different expression patterns of MMP genes (Table 2). MMP-1 (26), MMP-7 (26), MMP-10, MMP-14 and MMP-17 genes were only expressed in MKN-45 cells, while MMP-11, MMP-15 (26), MMP-23 and MMP-28 genes were expressed in both of the cell lines. On the other hand, MMP-3 (26), MMP-9 and MMP-12 gene expressions were determined in neither of them. Moreover, β-catenin expression was positive in both of the cell lines (Figure 2).
Full Table
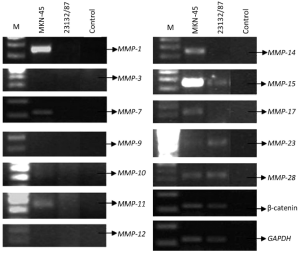
Effects of H2O2 exposure on expressions of MMP genes
Among the four MMP genes that were expressed in 23132/87 cells, only the expression of MMP-15 gene was determined to be increased following H2O2 exposure. However, in MKN-45 cells, there was an increase in the expressions of MMP-1, MMP-7, MMP-14, MMP-15, MMP-17, and β-catenin (Table 3). As seen in Figure 3, 12 h exposure to H2O2 resulted in a more increase in the expression of MMP-14 gene compared to 24 h exposure to H2O2. The same pattern was observed for MMP-7 gene as we have previously reported (27). Conversely, MMP-1, MMP-15 and MMP-17 exhibited more expression when MKN-45 cells were exposed to H2O2 for 24 h. β-catenin expression was also affected positively by the application of H2O2 to MKN-45 cells for 12 and 24 h.
Full Table
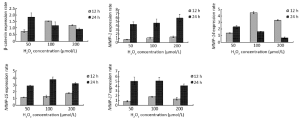
β-catenin expression was significantly 0.75 (80%), 1.60 and 1.45 fold increased (P<0.05) after 12 h of H2O2 exposure while it was 1.80, 1.45 and 0.90 (90%) fold increased significantly (P<0.05) after 24 h of oxidative stress treatment at 50, 100 and 200 µmol/L H2O2 concentrations, respectively, compared with the untreated control cells. Following 12 h of H2O2 application, MMP-14 displayed a significant up-regulation of 1.40, 4.40 and 2.03 fold (P<0.05) and 24 h of H2O2 application 2.45, 1.70 and 0.80 (80%) fold significant increase (P<0.05) at 50, 100 and 200 µmol/L H2O2 concentrations, respectively, compared with the untreated control cells. The analysis of the MMP-14 gene expression gave parallel patterns of expression profiles with β-catenin. There was a 0.76 (76%), 1.69 and 1.35 fold significant increase (P<0.05) after 12 h H2O2 exposure and 1.82, 1.33 and 0.92 (92%) fold significant increase (P<0.05) after 24 h H2O2 treatment at 50, 100 and 200 µmol/L H2O2 concentrations, respectively, compared with the control cells in MMP-14 gene (Figure 3). Likewise, MMP-7 gene showed a similar pattern of increase with different values at the same order of the concentrations (27).
The expression of MMP-1 gene in MKN-45 cells after 12 h of H2O2 exposure was nearly similar at all the three concentrations, but 24 h exposure to H2O2 caused a significant up-regulation of 4.6, 4.9 and 6.1 fold (P<0.05) at 50, 100 and 200 µmol/L concentrations, respectively. The same profile was observed in the MMP-15 and MMP-17 genes. Subsequent to 24 h H2O2 exposure, MMP-15 expression was significantly increased by 3.0, 3.9 and 3.4 fold (P<0.05) and MMP-17 expression was significantly up-regulated by 5.05, 5.10 and 4.30 fold (P<0.05) at 50, 100, and 200 µmol/L concentrations of H2O2, respectively (Figure 3).
Removal of oxidative stress with CAPE exposure
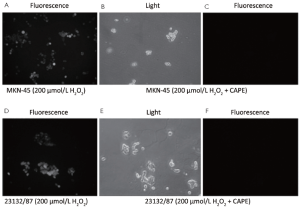
In both MKN-45 and 23132/87 cells, 24 h exposure to CAPE resulted in the removal of oxidative stress as shown by DCFH-DA method (Figure 4). And 200 µmol/L H2O2 exposure revealed an intracellular accumulation of oxidative stress in both of the cell lines (Figure 4A,D). However, the fluorescent microscopic view of the cells (Figure 4C,F) of which light microscopic views were demonstrated (Figure 4B,E) clearly reveals that CAPE treatment removed the accumulated oxidative stress in the viable MKN-45 and 23132/87 cells.
Effect of CAPE treatment on expressions of MMP genes compared to oxidative stress conditions
In MKN-45 cells, the expressions of MMP-1, MMP-7, MMP-14, MMP-15, MMP-17 and β-catenin genes that were up-regulated consequent to H2O2 exposure were down-regulated following inclusion of CAPE compared to the expression levels of the H2O2 exposed cells. Likewise, in 23132/87 cells, a decrease only in the expression of MMP-15 gene after CAPE application was observed since it was the only MMP in this cell line that was affected by H2O2 exposure (Table 3). β-catenin expression was 1.0 fold down-regulated significantly (P<0.05) following CAPE treatment compared to H2O2 treated MKN-45 cells, while it was 2.4 fold decreased significantly (P<0.05) in 23132/87 cells. Moreover, the MMP-1 and MMP-7 genes that were expressed in MKN-45 but not in 23132/87 cells showed a significant decrease 4.7 and 8.7 (P<0.05), respectively, in MKN-45 cells following CAPE treatment compared to the H2O2 exposed cells (Figure 5). Also, 1.3 and 10.8 fold significant decrease (P<0.05) in the expression of MMP-15 gene in MKN-45 and 23132/87 cells, respectively, was determined (Figure 5). The expressions of MMP-14 and MMP-17 genes that were only expressed in MKN-45 cells were decreased significantly by 11.20 and 0.38 (38%) fold (P<0.05), respectively, after the removal of oxidative stress. On the other hand, MMP-28 gene of which the expressions was demonstrated in both of the cell lines but not affected by H2O2 exposure, showed 1.20 and 0.27 (27%) fold significant decrease (P<0.05) in expression after CAPE treatment (Figure 5).

Discussion
Extensive research to date suggests that continued oxidative stress can lead to chronic inflammation, which in turn could mediate most chronic diseases such as diabetes, cardiovascular, neurological, pulmonary diseases and cancers. Oxidative stress is linked to various steps involved in carcinogenesis, including cellular transformation, promotion, survival, proliferation, invasion, angiogenesis and metastasis (16,17).
As a tumor promoter, it leads to increased levels of oxidatively modified bases such as 8-OHdG, in the tissues which are exposed to oxidative stress (28-32). Gastric tissue is the primary site where continuous oxidative stress and chronic inflammation appear. In this study, likewise, we demonstrated the presence of 8-OHdG, an oxidative stress induced DNA base modification, in MKN-45 and 23132/87 cells lines suggesting the involvement of oxidative stress in gastric tumor promotion with abnormality in their morphologies and detachment from the surface. We also showed that H2O2 exposure causes an accumulation of oxidative stress in the viable MKN-45 and 23132/87 cells and the removal of the oxidative stress with CAPE resulted in removal of the accumulated oxidative stress in these cells.
NF-κB, AP-1, P53, HIF-1α, PPAR-γ, β-catenin/Wnt, and Nrf2 are a variety of transcription factors which are activated by oxidative stress. The studies revealed that activation of these transcription factors can lead to the expression of over 500 different genes, including those for growth factors, cell cycle regulatory molecules, anti-inflammatory molecules, inflammatory cytokines, and chemokines (1,2,10,11). Here, we analyzed the change in the expression pattern of β-catenin, an adhesion molecule and a transcription factor, after H2O2 exposure of the gastric cancer cell lines, and we report that β-catenin expression increases in gastric cancer MKN-45 cells following H2O2 exposure but not in 23132/87 cells.
Among the studied MMPs, MMP-1, MMP-7, MMP-10, MMP-14 and MMP-17 genes were specifically expressed in MKN-45 cells. In these cells, parallel to β-catenin up-regulation following H2O2 treatment, a remarkable increase in the expressions of these MMP genes were also illustrated. Moreover, the removal of oxidative stress with CAPE caused a decrease in the expressions of the same genes. All these information suggests MMP-1, MMP-7, MMP-14 and MMP-17 up-regulation is directly related with the exposure to oxidative stress. In addition to this, increase and decrease in β-catenin expression under oxidative stress and non-oxidative stress conditions, respectively, might be related to the increase in the expressions of these MMP genes. On the other hand, MMP-11, MMP-15, MMP-23 and MMP-28 were expressed in both of the cell lines. Among these, the expressions of MMP-15 was increased after exposure of MKN-45 and 23132/87 cells to H2O2 while CAPE treatment reversed this effect and caused a decrease in MMP-15 gene expression in both of the cell lines studied, which also reveals the link between oxidative stress and MMP-15 gene expression. Interestingly, the down-regulation of MMP-28 expression in 23132/87 cells after CAPE treatment even though there was not an up-regulation following H2O2 exposure suggested an internal oxidative stress in this cell line prior to external H2O2 exposure took role in MMP-28 expression and removal of internal oxidative stress consequent to CAPE treatment resulted in the down-regulation of MMP-28 gene compared to the untreated control cells.
β-catenin-independent up-regulation of Wnt5A induced MMP-1 expression was observed in temporomandibular joint condylar chondrocytes (33). Here, we suggest that β-catenin might augment tumor invasion by increasing the rates of cell migration under oxidative stress conditions by promoting up-regulation of MMP-1 in gastric cancer MKN-45 cells. It is well established that MMP-7 is a Wnt-β-catenin target (34,35) and our results about the parallel up-regulation of β-catenin and MMP-7 following H2O2 exposure of MKN-45 cells is expected. Up-regulation of MMP-14 can also be related to the up-regulation of β-catenin since there exist data that demonstrated the detection of Fhit and β-catenin at the MMP-14 promoter in MCF-7 cells to regulate growth (36). On the other hand, there is no information about the regulation of MMP-17 by β-catenin, thus our results suggest a possible relation between the regulation of MMP-17 and β-catenin since under oxidative stress conditions, the expression of the genes increased, while the removal of oxidative stress caused the opposite.
In support of our data with change in the expressions of MMP genes, MMP-13, MMP-3 and MMP-10 were remarkably up-regulated by oxidant directly, and their activities were implicated in the invasive potential induced in NMuMG cells (37). Likewise, MMP-2 and MMP-9 were activated post-transcriptionally by prolonged oxidative treatment (38). The activation of MMPs, such as MMP-2, probably occurs by the reaction of ROS with thiol groups in the protease catalytic domain (39). In addition to their role as key regulators of MMP activation, ROS have been implicated in MMP gene expression (40). Both H2O2 and nitric oxide donors, as well as the increased expression of iNOS, stimulate the expression of several MMPs (MMP-1, MMP-3, MMP-9, MMP-10 and MMP-13) (40). In fibroblastic cells, the sustained production of H2O2 recently was shown to activate MMP-2 and to increase cell invasion (41). Oxidative stress may also modulate MMP expression by activation of Ras, or direct activation of the MAPK family members ERK1/2, P38, and JNK, or inactivation of phosphatases that regulate these proteins (42).
In conclusion, our data related to the change in the expressions of β-catenin and several MMP genes following oxidative stress exposure to gastric cancer cell lines give an idea about the effect of oxidative stress on the expressions of these genes which will be a hot spot research in gastric carcinogenesis at the molecular level in near future and take the attention of the researchers in this area since gastric mucosa is continuously exposed to oxidative stress and these genes are the main modulators of invasion and metastasis.
Acknowledgements
This project was granted by the Scientific and Technical Research Council of Turkey (TUBITAK) under the project number 105S352 (SBAG-K-110) and by the Scientific Research Fund of Fatih University under the project number P50030703.
Disclosure: The authors declare no conflict of interest.
References
- Reuter S, Gupta SC, Chaturvedi MM, et al. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 2010;49:1603-16. [PubMed]
- Cortes DF, Sha W, Hower V, et al. Differential gene expression in normal and transformed human mammary epithelial cells in response to oxidative stress. Free Radic Biol Med 2011;50:1565-74. [PubMed]
- D’Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 2007;8:813-24. [PubMed]
- Morel Y, Barouki R. Repression of gene expression by oxidative stress. Biochem J 1999;342:481-96. [PubMed]
- Dinkova-Kostova AT, Holtzclaw WD, Kensler TW. The role of Keap1 in cellular protective responses. Chem Res Toxicol 2005;18:1779-91. [PubMed]
- Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal 2005;7:385-94. [PubMed]
- Slupphaug G, Kavli B, Krokan HE. The interacting pathways for prevention and repair of oxidative DNA damage. Mutat Res 2003;531:231-51. [PubMed]
- Clopton DA, Saltman P. Low-level oxidative stress causes cell-cycle specific arrest in cultured cells. Biochem Biophys Res Commun 1995;210:189-96. [PubMed]
- Chua PJ, Yip GW, Bay BH. Cell cycle arrest induced by hydrogen peroxide is associated with modulation of oxidative stress related genes in breast cancer cells. Exp Biol Med (Maywood) 2009;234:1086-94. [PubMed]
- Seng S, Avraham HK, Jiang S, et al. The nuclear matrix protein, NRP/B, enhances Nrf2-mediated oxidative stress responses in breast cancer cells. Cancer Res 2007;67:8596-604. [PubMed]
- Almeida M, Han L, Martin-Millan M, et al. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem 2007;282:27298-305. [PubMed]
- Hoogeboom D, Essers MA, Polderman PE, et al. Interaction of FOXO with beta-catenin inhibits beta-catenin/T cell factor activity. J Biol Chem 2008;283:9224-30. [PubMed]
- Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol 2007;9:210-7. [PubMed]
- Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer 2003;3:276-85. [PubMed]
- Sanders LM, Henderson CE, Hong MY, et al. Pro-oxidant environment of the colon compared to the small intestine may contribute to greater cancer susceptibility. Cancer Lett 2004;208:155-61. [PubMed]
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7. [PubMed]
- Mantovani A. Cancer: Inflammation by remote control. Nature 2005;435:752-3. [PubMed]
- Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 2011;278:16-27. [PubMed]
- Farinati F, Cardin R, Cassaro M, et al. Helicobacter pylori, inflammation, oxidative damage and gastric cancer: a morphological, biological and molecular pathway. Eur J Cancer Prev 2008;17:195-200. [PubMed]
- Sternlicht MD, Bissell MJ, Werb Z. The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumor promoter. Oncogene 2000;19:1102-13. [PubMed]
- McManus MT, Sharp PA. Gene silencing in mammals by small interfering RNAs. Nat Rev Genet 2002;3:737-47. [PubMed]
- Ito E, Yana I, Fujita C, et al. The role of MT2-MMP in cancer progression. Biochem Biophys Res Commun 2010;393:222-7. [PubMed]
- Lowy AM, Clements WM, Bishop J, et al. beta-Catenin/Wnt signaling regulates expression of the membrane type 3 matrix metalloproteinase in gastric cancer. Cancer Res 2006;66:4734-41. [PubMed]
- Tang CH, Yamamoto A, Lin YT, et al. Involvement of matrix metalloproteinase-3 in CCL5/CCR5 pathway of chondrosarcomas metastasis. Biochem Pharmacol 2010;79:209-17. [PubMed]
- Bass DA, Parce JW, Dechatelet LR, et al. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol 1983;130:1910-7. [PubMed]
- Gencer S, Cebeci A, Irmak-Yazicioglu MB. Silencing of the MMP-3 gene by siRNA transfection in gastric cancer AGS cells. J Gastrointestin Liver Dis 2011;20:19-26. [PubMed]
- Gencer S, Irmak Yazicioğlu MB. Differential response of gastric carcinoma MKN-45 and 23132/87 cells to H2O2 exposure. Turk J Gastroenterol 2011;22:145-51. [PubMed]
- Wei H, Frenkel K. Suppression of tumor promoter-induced oxidative events and DNA damage in vivo by sarcophytol A: A possible mechanism of anti-promotion. Cancer Res 1992;52:2298-303. [PubMed]
- Hattori-Nakakukii Y, Nishigori C, Okamoto K, et al. Formation of 8-hydroxy-2-deoxyguanosine in epidermis of hairless mice exposed to near-UV. Biochem Biophys Res Commun 1994;201:1132-9. [PubMed]
- Ohshima H, Bartsch H. Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat Res 1994;305:253-64. [PubMed]
- Rosin MP, Saad el Din Zaki S, Ward AJ, et al. Involvement of inflammatory reactions and elevated cell proliferation in the development of bladder cancer in schistosomiasis patients. Mutat Res 1994;305:283-92. [PubMed]
- Weitzman SA, Gordon LI. Inflammation and cancer: role of phagocyte–generated oxidants in carcinogenesis. Blood 1990;76:655-63. [PubMed]
- Ge X, Ma X, Meng J, et al. Role of Wnt-5A in interleukin-1 beta-induced matrix metalloproteinase expression in rabbit temporomandibular joint condylar chondrocytes. Arthritis Rheum 2009;60:2714-22. [PubMed]
- Crawford HC, Fingleton BM, Rudolph-Owen LA, et al. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene 1999;18:2883-91. [PubMed]
- Brabletz T, Jung A, Dag S, et al. beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol 1999;155:1033-8. [PubMed]
- Weiske J, Albring KF, Huber O. The tumor suppressor Fhit acts as a repressor of beta-catenin transcriptional activity. Proc Natl Acad Sci USA 2007;104:20344-9. [PubMed]
- Mori K, Shibanuma M, Nose K. Invasive potential induced under long-term oxidative stress in mammary epithelial cells. Cancer Res 2004;64:7464-72. [PubMed]
- Westermarck J, Kahari V. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J 1999;13:781-92. [PubMed]
- Rajagopalan S, Meng XP, Ramasamy S, et al. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest 1996;98:2572-9. [PubMed]
- Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med 2004;37:768-84. [PubMed]
- Yoon SO, Park SJ, Yoon SY, et al. Sustained production of H(2)O(2) activates pro-matrix metalloproteinase-2 through receptor tyrosine kinases/phosphatidylinositol 3-kinase/NF-kappa B pathway. J Biol Chem 2002;277:30271-82. [PubMed]
- Storz P. Reactive oxygen species in tumor progression. Front Biosci 2005;10:1881-96. [PubMed]


