Analysis of factors influencing skip lymphatic metastasis in pN2 non-small cell lung cancer
Objective: Although many clinical studies on skip lymphatic metastasis in non-small cell lung cancer have
been reported, the risk factors for skip lymphatic metastasis are still controversy and debatable. This study
investigated, by multivariate logistic regression analysis, the clinical features of skip metastasis to mediastinal
lymph nodes (N2) in non-small cell lung cancer (NSCLC) patients.
Methods: We collected the clinicopathological data of 256 pN2-NSCLC patients who underwent
lobectomy plus systemic lymph node dissection in Fujian Medical University Union Hospital. The cases
in the present study were divided into two groups: skip metastasis (N2 skip+) and non- skip metastasis
(N2 skip-). A retrospective analysis of clinical pathological features of two groups was performed. To
determine an independent factor, multivariate logistic regression analysis was used to identify possible
risk factors.
Results: A total of 256 pN2-NSCLC patients were recruited. The analysis results showed that gender,
pathologic types, surgery, pleural involvement, smoking history, age, tumor stages, and differentiation were
not statistical significant factors impacting on skip metastasis in pN2-NSCLC (P>0.05), whereas tumor size
was an independent factor for skip metastasis (P=0.02).
Conclusions: The rate of skip lymphatic metastasis increases in pN2-NSCLC patients, in accompany with
an increased tumor size.
Key words: Lung cancer; lymph node; skip metastasis
Introduction
Non-small cell lung cancer (NSCLC) constitutes about 85% of all newly diagnosed cases of lung cancer and continues to be the leading cause of cancer-related deaths worldwide (1,2). The majority of patients present with either locally advanced or metastatic disease and only 20-30% of patients have potentially operable, early stage disease at presentation (3,4). It is known as skip mediastinal lymph node metastasis that lung cancer metastasis to mediastinal lymph node (N2) occurs without involvement of pulmonary or hilar lymph node (5). Mediastinal skip lymphatic metastasis is important for mediastinal lymph node dissection (MLND), because it is an important basis for a reasonable range of MLND (6). N2 skip metastasis is considered as an independent subtype within the N2 metastasis (7) and its exact mechanism is currently not clear. The argument regarding risk factors for skip lymphatic metastasis in lung cancer is mainly focused on pathology as well as the localization, stages, and size of tumors. To further explore the factors influencing skip lymphatic metastasis in NSCLC, a retrospective analysis of 256 NSCLC cases were performed in our current study.
Patients and methods
Patients
From January 2001 through January 2012, 256 patients underwent lobectomy plus systemic lymph node dissection for NSCLC in Fujian Medical University Union Hospital. These patients aged 29-80 years [(58.5±9.6) years]. Tumors were located at the upper lobe(n=138, 53.9%), middle lobe (n=13, 5.1%), lower lobe(n=99, 38.7%) and pulmonary hilum (n=6, 2.3%). The pathological types included adenocarcinoma (n=141, 55.1%), squamous cell carcinoma (n=90, 35.2%), adenosquamous cell carcinoma (n=11, 4.3%), large cell carcinoma of lung (n=9, 3.5%), and other primary lung cancers (n=5, 2%) that included atypical carcinoid (n=3), epidermoid carcinoma (n=1), and sarcomatoid carcinoma (n=1). The size of tumors ranged 10-150 mm, with an average size of (47.5±21.6) mm. In 256 cases, the total N1 lymph nodes for pathological examination was present at least 1 group/case, and up to 4 groups/case, with an average number (2.3±0.8) groups/case. Among the 256 cases, there were 44 cases of skip lymphatic metastasis, which were at least 2 groups/case, and up to 4 groups/case, with an average number (2.4±0.6) groups/case. The other 212 cases non-skip metastasis, which were at least 1 group/case, and up who 4 groups/case, with an average number (2.3±0.8) groups/case. Before surgery, all patients were naive to chemotherapy, without previous cancer history, and with only one localized tumor that had no distant metastasis. In cases that were not suspected of skip metastasis, the number of N1 lymph nodes for examination was required not less than 2 group/case and the related examinations were carried out to exclude surgery contraindications.
Surgery
The metastatic lymph nodes in lung cancer were removed by systemic lymph node dissection, according to Naruke’s system, included N1 and N2 lymph nodes, the 2nd, 3rd, 4th, 7th, 8th, 9th groups/stations of mediastinal lymph nodes in right lung cancer or the 4th-9th stations of mediastinal lymph nodes in left lung cancer. The detail information on the location, station and number of resected lymph nodes was shown in Table 1. TNM classification for NSCLC in this study was based on the revised staging classification for lung cancer issued by UICC/AJCC in 2009, while the pathologic typing of lung cancer was made, according to 2004 WHO classification system for primary lung cancer.
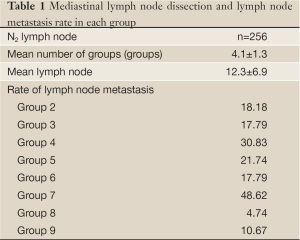
Full table
Statistical analysis
Two-sided test was used in all statistical tests and P<0.05 was considered statistically significant. Followed by univariate analysis of possible risk factors, multivariate analyses were performed by logistic regression method. Statistical analysis was performed using SPSS 13.0 software (SPSS Inc. Chicago, Illinois, USA).
Results
Univariate analysis of possible risk factors influencing N2 skip metastasis
In order to determine the possible factors influencing N2 skip metastasis in NSCLC, univariate analysis with Chi square test were performed in our study, in which there were 6 cases that tumor was found in pulmonary hilum and in more than one lobe of the same lung or in other lung. However, these 6 cases were excluded in Chi square test for the factor regarding tumor location. The analysis showed that there were no statistically significant (P>0.05) differences in gender, age, smoking, tumor location, pathology, surgery, pleural involvement, and differentiation between groups of N2 skip+ and N2 skip-. The only one significant difference was tumor size (as shown in Table 2).
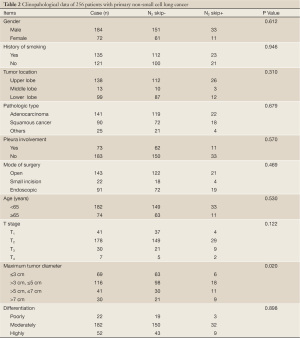
Full table
Multivariate analyses using logistic regression method
The major aim of this study is to identify the factors, based on clinical pathological characteristics of NSCLC, which are correlated with skip metastasis of NSCLC. However, the results of univariate analysis revealed that the factors regarding gender, age, smoking history, pathology, differentiation, tumor stage, surgery, tumor location and pleural involvement were not significantly correlated with skip metastasis in NSCLC. Only the factor regarding tumor size was found statistically significant in the test. In the multivariate analysis, there were two candidates for our consideration, i.e. two highly relevant factors: tumor size and tumor stage. The former, tumor size was finally chosen for multivariate analysis, because only one potential factor can be included in the analysis. Precluding the confound of independent variables, multivariate analysis was performed, using logistic regression model, by taking tumor location and tumor size as covariant and skip metastasis as a dependent variable. The results in Table 3 show that in patients with clinical N2 NSCLC, tumor size was an dependent factor influencing N2 skip metastasis, but tumor location was not statistically significantly associated with N2 skip metastasis (P>0.05).

Full table
Discussion
Misthos et al. considered that squamous cell carcinomas are most prone to skip metastasis (8). Gunluoglu et al. reported that patients with adenocarcinoma were easier to have skip lymphatic metastasis (9). In our current study, we found that the difference of pathological classification between the two groups was not statistically significant, and perhaps pathological type was not an independent factor influencing skip metastasis.
Whether the location of the primary tumor is associated with N2 skip metastasis is currently still disputed. Melfi et al. argued that the incidence of N2 skip metastasis was highest in right lower lobe of lung cancer (10). Our study found that the incidence of N2 skip metastasis was higher in tumors that occurred in either upper lobe or middle lobe on both sides of the lung. This finding is consistent with Riquet’s report (11). However, the difference is not statistically significant, due to limited case number and larger variations of tumor volume between the tumors in different locations as well as other involved mechanisms such as a larger variation in the channel and modes of lymphatic return between pulmonary lobes and mediastinal lymph nodes.
Riquet et al. found that there was subpleural lymphatic channel that was directly linked to mediastinal lymph nodes (11). They think it is a reason why skip mediastinal lymphatic metastasis occurred in the patients with NSCLC. Furthermore, the pulmonary lymphatic channels also directly connect the bottom of lower lobes to upper lobes of the lung. The lymphatic drainage from interlobular lymph nodes to hilar lymph nodes, also directly to mediastinal lymph nodes, is mainly through the widespread subpleural lymphatic network, suggesting the anatomic basis for N2 skip metastasis. Accordingly, if there is pleura involvement in these NSCLC patients, will the incidence of skip mediastinal lymphatic metastasis be even higher? It is unclear whether those patients with pleura involvement have a greater chance of shedding of tumor cells into the pleural cavity, thereby leading to an increase in skip mediastinal lymphatic metastasis. Since there are no statistically significant (P>0.05) differences between pleura involvement and skip lymphatic metastasis in our study, we consider that the pleura involvement is not an independent factor influencing skip metastasis.
Whether the tumor size is correlated with skip mediastinal lymphatic metastasis is currently still disputed. Our study revealed that an increased tumor size increased the incidence of skip lymphatic metastasis in NSCLC patients, which is consistent with Riquet’s results (12). In their study, the analysis of the correlation of tumor stage or size respectively with skip metastasis indicated that in the test, tumor stage had a P value of 0.1, while tumor size had a P value of 0.0079. However, in the report of Misthos et al. (8), the incidences of skip lymphatic metastasis in T1 and T2 were significantly higher than that in T3. Benoit et al. also found that the case with higher tumor stage had a higher rate of skip lymphatic metastasis (13). However many reports showed that the correlation of tumor stage and N2 skip metastasis is not statistically significant. As we know, tumor staging actually includes several factors regarding tumor size, plural involvement, and involvement of important structures. Therefore, tumor size is not equal to tumor stage. We think that only tumor size that is also a parameter for tumor staging contributes to skip metastasis. If the analyses of skip metastasis are based on the data of tumor stage, the results are less reliable. Tumor size or tumor stage, which one is actually main factor influencing skip metastasis? What is the cause of the larger tumor with a higher risk for N2 skip metastasis? The following factors should be considered: the larger tumor size or volume, the more interlobular lymphatic channels around the tumor, as well as higher risk for the metastasis of tumor cells into subpleural lymphatic channel through interlobular lymphatic channel. Previous studies found that the presence of subpleural lymphatic channels that are directly linked to mediastinal lymph nodes, increased the risk of N2 skip metastasis in larger tumor, although the tumor metastasis through the lymphatic channels that are accompanied by bronchus, artery, and vein is not increased.
In the literatures (14,15), the percentage of patients with skip metastasis is about 13% to 42% of total N2 patients; similarly, it was 17.2% in our study. The rates of N2 skip lymphatic metastasis varied in the different reports, possibly due to the missed diagnosis of hilar lymph node micrometastasis. However, Nl lymph node micrometastasis might be not detected by conventional pathological examination. It is reported that the rates of missed diagnosis for pulmonary or hilar lymph node micrometastasis were around 20% (16). Passlik et al. reported approximately 20% Nl lymph node micrometastasis detected by immunohistochemical methods (17). Using RT-PCR assay, Wu et al. investigated the expression of LUNX, a specific gene related to lung cancer, in mediastinal lymph nodes (18). They found that the percentage of the lymph nodes that are LUNX-mRNA positive, were up to 62.5% in the patients with stage III of lung cancer, suggesting very high rate of mediastinal lymph node micrometastasis in the patients with NSCLC that underwent surgery. Meanwhile, this phenomenon may also be caused by the absence of intrapulmonary lymph nodes (12-14 groups/stations) in the specimens for pathological examination. The specimen of N1 lymph nodes of the case with skip metastasis for pathological examination had at least 2 groups/case in our study, thus it would be better to reduce such effect. Moreover, studies also found that pN0 lymph node micrometastases might be associated with the stage, size, and location of tumors, in which there were a part of cases with skip metastasis (19), indicating the similarity in clinical pathological features between pN0 lymph node micrometastases and N2 skip metastasis. Therefore, we think that N2 skip metastasis research will be benefited from the studies on pN0 lymph node micrometastases.
Current studies suggest that the patient with skip metastasis has a better survival rate, but the underlying mechanism is not clear. Benoit et al. believed that less group number of the positive mediastinal lymph nodes is a reasonable explain for better prognosis of skip metastasis (13); in other words, the duration of survival for the patients with skip metastasis depends on the group number of the positive N2 lymph nodes. N2 skip lymphatic metastasis may be an earlier stage of lymphatic metastasis in NSCLC and its significance is similar to Nl lymph node metastasis. In addition, some scholars proposed that dysregulation of gene expression associated with primary tumor is one of the most important factors influencing lymph node metastasis (20). Prenzel et al. found that decreased expression of Bcl-2 and elevated expression of P21 were associated with N2 skip metastasis in lung cancer (21). Whether there are more aberrant biological changes in the cases with N2 skip metastasis positive than that in N2 skip metastasis negative cases is still unclear.
In conclusion, the rate of skip lymphatic metastasis increases in pN2-NSCLC patients, in accompany with an increased tumor size.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Metro G, Crinò L. Advances on EGFR mutation for lung cancer. Transl Lung Cancer Res 2012;1:5-13.
- Hennon MW, Demmy TL. Video-assisted thoracoscopic surgery (VATS) for locally advanced lung cancer. Ann Cardiothorac Surg 2012;1:37-42.
- Ellis PM, Vandermeer R. Delays in the diagnosis of lung cancer. J Thorac Dis 2011;3:183-8.
- Yang P, Xu XY, Liu XJ, et al. The value of delayed 18F FDG-PET imaging in diagnosis of solitary pulmonary nodules: A preliminary study on 28 patients. Quant Imaging Med Surg 2011;1:31-4.
- Tateishi M, Fukuyama Y, Hamatake M, et al. Skip mediastinal lymph node metastasis in non-small cell lung cancer. J Surg Oncol 1994;57:139-42.
- Okada M, Tsubota N, Yoshimura M, et al. Proposal for reasonable mediastinal lymphadenectomy in bronchogenic carcinomas: role of subcarinal nodes in selective dissection. J Thorac Cardiovasc Surg 1998;116:949-53.
- Yoshino I, Yokoyama H, Yano T, et al. Skip metastasis to the mediastinal lymph nodes in non-small cell lung cancer. Ann Thorac Surg 1996;62:1021-5.
- Misthos P, Sepsas E, Athanassiadi K, et al. Skip metastases: analysis of their clinical significance and prognosis in the IIIA stage of non-small cell lung cancer. Eur J Cardiothorac Surg 2004;25:502-8.
- Gunluoglu Z, Solak O, Metin M, et al. The prognostic significance of skip mediastinal lymphatic metastasis in resected non-small cell lung cancer. Eur J Cardiothorac Surg 2002;21:595.
- Melfi FM, Chella A, Menconi GF, et al. Intraoperative radioguided sentinel lymph node biopsy in non-small cell lung cancer. Eur J Cardiothorac Surg 2003;23:214-20.
- Riquet M, Hidden G, Debesse B. Direct lymphatic drainage of lung segments to the mediastinal nodes. An anatomic study on 260 adults. J Thorac Cardiovasc Surg 1989;97:623-32.
- Riquet M, Assouad J, Bagan P, et al. Skip mediastinal lymph node metastasis and lung cancer: a particular N2 subgroup with a better prognosis. Ann Thorac Surg 2005;79:225-33.
- Benoit L, Anusca A, Ortega-Deballon P, et al. Analysis of risk factors for skip lymphatic metastasis and their prognostic value in operated N2 non-small-cell lung carcinoma. Eur J Surg Oncol 2006;32:583-7.
- Casali C, Stefani A, Natali P, et al. Prognostic factors in surgically resected N2 non-small cell lung cancer: the importance of patterns of mediastinal lymph nodes metastases. Eur J Cardiothorac Surg 2005;28:33-8.
- Prenzel KL, Baldus SE, Mönig SP, et al. Skip metastasis in nonsmall cell lung carcinoma: predictive markers and isolated tumor cells in N1 lymph nodes. Cancer 2004;100:1909-17.
- Prenzel KL, Mönig SP, Sinning JM, et al. Role of skip metastasis to mediastinal lymph nodes in non-small cell lung cancer. J Surg Oncol 2003;82:256-60.
- Passlick B, Izbicki JR, Kubuschok B, et al. Detection of disseminated lung cancer cells in lymph nodes: impact on staging and prognosis. Ann Thorac Surg 1996;61:177-82; discussion 183.
- Wu YL. Multidisciplinary combination therapy for lung cancer. Zhonghua Jie He He Hu Xi Za Zhi 2007;30:81-2.
- Jabbour SK, Daroui P, Moore D, et al. A novel paradigm in the treatment of oligometastatic non-small cell lung cancer. J Thorac Dis 2011;3:4-9.
- Li YQ, Shi AH, Li FH, et al. Phase I study to determine MTD of docetaxel and cisplatin with concurrent radiation therapy for stage III non-small cell lung cancer. Chin J Cancer Res 2011;23:129-33.
- Prenzel KL, Mönig SP, Sinning JM, et al. Role of skip metastasis to mediastinal lymph nodes in non-small cell lung cancer. J Surg Oncol 2003;82:256-60.


